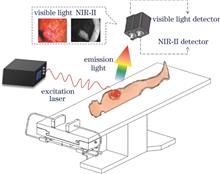
Real-time visualization of tumor boundaries and occult lesions is essential for precision surgery. However, conventional imaging struggles to meet intraoperative navigation demands. Second near-infrared region (NIR-II, 1000-1700 nm) fluorescence imaging enables high precision surgical navigation through low scattering effect, high sensitivity, spatial resolution, and real-time capability. Recent innovations in probes, methods, and systems accelerate NIR-II clinical translation. Targeted probes enhance tumor specificity, tomography reconstruction and deep learning enable 3D lesion localization and pathological correlation, open and endoscopic systems support diverse surgical scenarios. Preliminary clinical studies demonstrate significant value for intraoperative tumor imaging and neurovascular preservation. This paper reviews the technological evolution and clinical transformation challenges of NIR-II surgical navigation, focuses on the development status and technical difficulties of imaging probes, methods, systems and clinical applications, and proposes the future development direction of interdisciplinary collaborative promotion of high-sensitivity imaging probes, tumor imaging and quantitative analysis methods, and the intelligent surgical navigation system, in order to improve the clinical transformation speed and application potential of fluorescent surgical navigation technology.
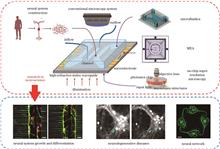
The optoelectronic integrated lab-on-chip overcomes the limitations of traditional optoelectronic technologies, providing new research tools for exploring the mechanisms of neurological diseases, neural repair, and neural network intelligent computing. To better promote neuroscience research based on the optoelectronic integrated lab-on-chip, the paper provides an overview of the technological development for optoelectronic integrated lab-on-chip and offers a comprehensive comparative analysis of the technical details. Furthermore, it discusses the applications of the optoelectronic integrated lab-on-chip in the research of neural system growth-differentiation-maturation, optoelectronic stimulation of neurodegenerative diseases, and functional regulation of neural networks, confirming the reliability and advanced nature of the platform in neuroscience research. Finally, this paper summarizes the current challenges in research and prospects future research directions.
Fluorescence imaging has emerged as a widely adopted technique for in vivo biological studies due to its noninvasive nature, high specificity, and exceptional spatiotemporal resolution. Super-resolution microscopy transcends the diffraction limit, achieving spatial resolutions of tens of nanometers or better, enabling visualization of intricate intracellular structural changes. These methodologies demonstrate significant potential for examining the living brain, particularly in studying neuronal activities. This review examines recent developments in stimulated emission depletion microscopy (STED), structured illumination microscopy (SIM), single-molecule localization microscopy (SMLM), and super-resolution optical fluctuation imaging (SOFI) for in vivo imaging, addressing key challenges including imaging depth, phototoxicity, motion artefacts and three-dimensional reconstruction, while exploring imaging quality enhancement strategies such as multi-photon excitation, near-infrared imaging, adaptive optics, and deep-learning-based approaches. Additionally, it presents insights into the future trajectory of in vivo super-resolution imaging in neuroscience and clinical diagnostics.
Light-field microscopy has demonstrated remarkable potential in biomedical imaging. However, its inherent challenges—such as uneven spatial sampling and reconstruction artifacts—have limited its broader application. To address these issues, this paper introduces the recent advancements in Fourier light-field microscopy (FLFM). By recording light field information in the Fourier domain, FLFM achieves a consistent aliasing of both spatial and angular components of incident light. This innovative approach effectively mitigates reconstruction artifacts and enhances the uniformity of imaging resolution. In this work, we provide a comprehensive explanation of the imaging principles, system designs, and reconstruction algorithms of FLFM, comparing its point spread function to that of conventional light-field microscopy. Furthermore, we explore the application of FLFM in biological research, particularly in subcellular imaging and model organism studies. Finally, we offer insights into the future directions of FLFM.
Single-molecule tracking (SMT) imaging technology has become a crucial tool in modern biophysics and cell biology, achieving significant advancements in recent years. By monitoring the dynamic behavior of individual molecules in real time, this technique reveals microscopic processes and transient events that are often obscured by traditional ensemble-averaged methods. This paper provides a comprehensive review of the latest research developments in the field of SMT imaging, with a particular focus on three-dimensional (3D) tracking methods based on closed-loop feedback mechanisms. These methods are primarily categorized into structured signal detection and structured excitation scanning, including techniques such as tetrahedral detection, split detection, tetrahedral excitation, orbital scanning, 3D dynamic photon localization, extremum search localization, minimum cross-entropy localization, and asynchronous readout SPAD arrays. The paper also briefly introduces image acquisition-based closed-loop feedback 3D tracking schemes. We systematically compare and analyze various methods in terms of spatial resolution, temporal resolution, tracking range, system stability, and implementation complexity, aiming to provide guidance for technology selection under different application scenarios. Furthermore, the paper explores integration strategies between 3D tracking and environmental background imaging, as well as multimodal fusion approaches combining optical parameters such as fluorescence lifetime, fluorescence spectrum, and polarization to achieve complementary information for in-depth analysis of complex biological events. Finally, the paper discusses future development directions, including multi-target parallel tracking, deep-tissue imaging, AI-assisted closed-loop control, system miniaturization and integration, and photobleaching suppression. The potential applications of these technologies in cancer biology, neuroscience, virology, and other biomedical fields are also explored. This review aims to provide a systematic reference and theoretical foundation for the continued development and practical deployment of single-molecule tracking technologies.
Optical imaging has the advantages of real-time monitoring, non-invasive, and high spatial-temporal resolution, and has been widely used in the biomedical field. Afterglow luminescence refers to the phenomenon that the material can continue to emit visible light after stopping illumination. Based on this, afterglow imaging can significantly reduce the interference of spontaneous fluorescence in biological tissues, and become an important means to improve the imaging quality, signal-to-noise ratio, and sensitivity. In this paper, the main categories of organic afterglow luminescent materials mediated by reactive oxygen species are systematically reviewed, and their luminescent mechanisms are discussed. The applications of these materials in biomedical detection, imaging, and tumor treatment are comprehensively introduced.
Optical microscopic imaging technology can provide subcellular structural and functional information in a non-invasive way, and has gradually become the key technology of basic biological research. As the only window that can directly and noninvasively observe the microvascular and neural structure, the eye is of great significance in the study of visual function, cardiovascular, metabolic, and neurodegenerative diseases. This paper briefly introduces the current commonly used eye imaging technology and its application in the research of eye diseases, analyzes its advantages, limitations and development trend, and discusses the great potential of adaptive optics and artificial intelligence in improving imaging quality and assisting pathological analysis, so as to provide reference for future technology development, basic scientific research, and clinical application.
After more than 20 years of development, miniature two-photon microscope and endomicroscope technology have shown important application value in the field of in situ three-dimensional pathological detection and brain imaging of free walking animals (such as mice). In this paper, centering on two representative technological approaches—fiberscanning two-photon endomicroscope and head-mountable two-photon microscope, the core principles and methods of the miniaturized two-photon (endo)microscope imaging system are systematically described, focusing on the following key technical challenges and solutions: 1) efficient delivery and focusing of excitation light; 2) optimized collection of backscattered signals; 3) miniaturized distal-end scanning mechanism. Finally, the representative applications of this technology in neuroscience and clinical medicine are summarized, and its future development trends are briefly discussed.
In recent years, head-mounted miniaturized microscopy technology has witnessed transformative breakthroughs in neuroscience research through continuous innovation, shifting from instrumental advancement to scientific discovery. Characterized by its miniaturized structure, single-cell resolution, and long-term stable imaging capabilities, this technology successfully overcomes the spatial limitations of traditional fixed-microscopy systems, offering a revolutionary observation window for dynamic analysis of neural circuits in freely behaving animals. Technical developments have followed three main trends: single-photon fluorescence microscopes enable multi-region synchronization through optical optimization; multiphoton microscopes break through deep-tissue imaging barriers with miniaturized scanning modules; and multimodal integrated systems combine optogenetics, electrophysiology, and photoacoustic imaging to advance neurovascular coupling research. At the scientific application level, this technology has expanded to cover decoding neural encoding mechanisms in natural behavioral contexts, in vivo pathological tracking of neurological disease models, drug screening and pharmacological evaluation, and development of closed-loop brain-computer interfaces driven by neural signals. This review systematically summarizes the core advancements in head-mounted microscopy technology and its applications in brain science research, analyzes current technical bottlenecks, and anticipates future technological directions and applications based on emerging technologies such as wireless data transmission and charging, deep learning computational imaging, voltage imaging, and multimodal data fusion. It provides theoretical frameworks and technical roadmaps for the development of next-generation neural observation tools.
The event-driven microscopy refers to a range of biological intravital microscopic imaging techniques that integrate optimized optical illumination, imaging, and sensing systems, along with advanced image processing algorithm and real-time feedback mechanisms. This approach enables the real-time monitoring and recording of dynamic activities while also facilitating the identification of specific events for optimizing imaging parameters or switching between different imaging modes. Consequently, it enhances both the resolution of imaging and system efficiency. Compared to conventional microscopes, the utilization of event-driven microscopes not only minimizes photo damage to biological samples, facilitating long-term stable observation of living activities, but also offers a distinct advantage in large-scale data processing. As a result, this technology has gained widespread application in the research of biological processes at the cellular and subcellular scales. This review provides an overview of the development history and classifications pertaining to event-driven intravital microscopy. Furthermore, we explore their wide-ranging applications in the investigation of neuroscience, cancer metastasis, and nanomedicine. Subsequently, we discuss limitations within this technology domain, and anticipate immense potential for event-driven microscopy in elucidating intricate biological dynamics and fostering advancements in precision medicine.
Optical coherence tomography (OCT) enables non-destructive, non-invasive acquisition of high-resolution cross-sectional images of samples. Improving imaging speed has been one of the most critical research directions in OCT technology development. Line-field (LF) OCT technology offers a high-speed imaging solution by illuminating samples with line focusing and implementing parallel detection. Compared to traditional point-focusing OCT, LF-OCT can increase imaging speed by tens of times while maintaining phase stability and power safety advantages. Consequently, it has found widespread applications in biomedical and industrial inspection fields. This paper reviews the developmental history of LF-OCT, focusing on its general system architecture and significant technological innovations, along with major application cases across various domains. The advantages and limitations of LF-OCT are thoroughly analyzed, and effective improvement measures are proposed. Looking forward, LF-OCT is expected to expand its applications in functional imaging and industrial inspection. Particularly in dermatology, it shows promise to replace traditional point-focusing two-dimensional scanning OCT as the mainstream technology.
Optical surface waves, owing to their label-free nature and high sensitivity, have found extensive applications in the biomedical field. Bloch surface waves (BSW), which do not require metallic media, exhibit superior characteristics such as low absorption loss and high detection sensitivity, making them highly promising for biomedical applications. This paper introduces the generation principles and excitation methods of BSW detection technology, covering technical features and research progress in prism coupling, grating coupling, optical fiber coupling, and waveguide coupling. It focuses on the current application status of BSW in two major domains: biomedical sensing and imaging. For sensing applications, the paper analyzes breakthrough advances in label-free detection, fluorescence-enhanced detection, and dual-mode detection. For imaging applications, it elaborates on their label-free high-resolution imaging capabilities as well as the key mechanism for achieving super-resolution imaging through integration with structured illumination microscopy. Finally, future development directions for BSW technology are envisioned, emphasizing sensitivity enhancement and multimodal integration.
Urinary system tumor is one of the important parts of global cancer, and surgery is still the main way to treat this kind of cancer. Although the tumor tissue can be removed as much as possible through visual observation and tactile feedback during the operation, there are still some small lesions that are difficult to be recognized by the naked eye and small tumors disseminated during the operation, which cannot be effectively removed. Fluorescent molecular imaging (FMI), as a new imaging technology, can accurately locate the lesions during surgery, provide intraoperative navigation function, and achieve precise surgery. The development of fluorescent molecular probes for intraoperative navigation is of great significance to improve the prognosis of patients with urinary system tumors and reduce the recurrence rate. This paper reviews the application status of fluorescent molecular probes in urinary system tumors at home and abroad, and analyzes the current research progress and future development direction.
Mitochondria are the core organelles of cell energy metabolism and homeostasis regulation, and are closely related to neurodegenerative diseases, cancer and metabolic diseases. Accurate detection of mitochondrial markers is essential for early diagnosis, mechanism research and curative effect evaluation of disease. Near-infrared second window (NIR-II, 1000?1700 nm) fluorescence imaging technology has become a leading tool for real-time dynamic monitoring of mitochondrial markers with the advantages of deep tissue penetration, low autofluorescence interference and high temporal and spatial resolution. This paper systematically reviews the research progress of NIR-II fluorescent probe in the detection of mitochondrial markers, including the detection strategies of reactive oxygen species (ROS), membrane potential (MMP), microenvironment pH and metabolic molecules [ATP, NAD(P)h, etc.]. The related probes targeted mitochondria specific structures (such as triphenylphosphine cation) to locate mitochondria, and combined with ratio design, FRET/BRET energy transfer and other strategies to improve the detection sensitivity and signal-to-noise ratio. For example, Mito-Cy-Tfs (ROS detection), MOF based ATP response probe and pH sensitive Fe-CDs have all achieved accurate visualization of markers in living cells and in vivo. Finally, the challenges faced by the NIR-II fluorescent probe, its future prospects, and the ideas and obstacles for its clinical transformation are analyzed.
Photoacoustic imaging has demonstrated great potential in clinical diagnosis and treatment monitoring, with the use of exogenous contrast agents further expanding its imaging capabilities. In recent years, significant progress has been made in the research on liposomal photoacoustic contrast agents. This review analyzes the major challenges currently facing liposomal photoacoustic contrast agents, including issues related to stability and in vivo safety. It further discusses the preparation methods of liposomes, surface modification strategies, and their biomedical applications. Finally, the future development direction of liposomal photoacoustic contrast agents is discussed.
Ultrasound diagnostics holds significant clinical value in examinations across multiple organ systems due to its non-invasive, safe, and portable advantages, though its efficacy remains highly operator-dependent. Ultrasound robot addresses this limitations by leveraging robotic arms to achieve precise probe control, reducing operator workload while enhancing procedural standardization, thereby offering a novel pathway to elevate healthcare quality. This paper systematically outlines the technical architecture of ultrasound robot systems, with a focus on breakthroughs in core technologies such as scanning path planning, contact force control, and probe posture optimization. By integrating advancements in deep reinforcement learning and embodied intelligence large models, the study explores the potential of intelligent algorithms in autonomous navigation and precision operation. Furthermore, it summarizes the clinical applications of ultrasound robot in standardized image acquisition, abdominal/cardiovascular/obstetric imaging, and minimally invasive surgery navigation. As core technologies continue to advance, ultrasound robot systems are poised to drive the intelligent transformation of medical imaging, providing robust support for precision medicine and clinical decision-making, ultimately propelling healthcare services toward unprecedented excellence.
Near-infrared II (NIR-II) fluorescence imaging technology has emerged as a research hotspot in the field of biomedical imaging due to its advantages including deep tissue penetration, high resolution, and low background interference. However, traditional probes still face challenges in terms of penetration depth, resolution, signal-to-noise ratio, real-time performance, and safety. This study comprehensively reviews the research progress of inorganic and organic NIR-II fluorescent probes, including the design strategies, performance optimization, and applications in in vivo imaging, tumor diagnosis and therapy, and vascular visualization of single-walled carbon nanotubes (SWCNTs), quantum dots (QDs), rare-earth nanoparticles (RENPs), gold nanoclusters (AuNCs), as well as organic small-molecule dyes. Through molecular engineering, surface modification, and material innovation, researchers have successfully enhanced the quantum yields, biocompatibility, and targeting capabilities of these probes, and developed high-performance probes for the NIR-II window. This study aims to provide guidance for the further development of NIR-II fluorescent probe technology and its clinical application, as well as to explore its future directions.
Using multicolor autofluorescent plant pollen grains and flower buds as samples, the performance of confocal laser scanning microscopy (CLSM) and optical sectioning structured illumination microscopy (OS-SIM) are compared under similar imaging conditions for multi-channel 3D imaging. Results demonstrate that OS-SIM achieves one order of magnitude faster imaging speed than conventional single-point scanning CLSM due to its wide-field imaging mechanism. Additionally, OS-SIM reduces photodamage by three orders of magnitude by employing LED illumination instead of laser excitation. The modular design of OS-SIM also lowers system costs to less than half that of CLSM. This study provides a more cost-effective solution for multicolor live-cell fluorescence imaging, highlighting technical and economic advantages of OS-SIM for applications such as dynamic 3D cell tracking and neural activity monitoring.
A stimulated Raman scattering (SRS) technique-guided method for visualizing and evaluating in vitro cellular inflammation models is proposed, which provides new ideas for drug evaluation. A single-cell feature extraction process is established to obtain a total of 10 features including the morphology and composition of cells, and cells are segmented using spectral phasor analysis extraction to obtain the spectral information of different cellular regions. To validate the feasibility of the described method, an inflammatory cell model is constructed. Different mass concentrations of lipopolysaccharide (LPS) are used to induce phenotypic changes in RAW264.7 cells, accompanied by varying degrees of inflammatory responses. Results show that the cell area of the LPS-treated group is significantly larger (P<0.01) compared with the control group, and the ratio of intensity between 2855 cm-1 and 2940 cm-1 wave number in LD spectra is significantly increased (P<0.0001) and correlated with the mass concentration of LPS. The proposed method can realize the accurate extraction of single-cell feature information, which can be successfully applied to the differentiation of LPS-induced models with different degrees of inflammation, and verifies the applicability of SRS technology in the visual assessment of in vitro cellular models.
Lipids play a crucial role in the development of metabolic diseases. However, traditional imaging techniques still face challenges such as low sensitivity and insufficient penetration depth in the specific imaging of lipid tissues. To improve lipid imaging capabilities and meet the needs of clinical translation, we develop a handheld multispectral photoacoustic imaging probe integrated with D?O coupling and multiwavelength excitation, enabling label-free and high-sensitivity lipid detection through non-negative matrix factorization-based spectral unmixing. Comparative experiments on oil sample in the tube, ex vivo adipose tissues, and pork belly phantom demonstrate that the signal-to-noise ratio of lipid images at 1210 nm improves by approximately 33 dB over 930 nm. Lipid signal intensities are 4.9?6.0 times that of the 930 nm band. In in vivo imaging of mouse abdominal fat, the system achieves a noninvasive imaging depth exceeding 1.5 cm, revealing clear and accurately localized lipid distributions in complex tissue environments. This work represents the implementation of D2O coupling in a portable probe, substantially improving photoacoustic signal transmission efficiency at high-absorption near-infrared wavelengths. The system offers a lightweight and practical solution for lipid imaging, providing a novel technical pathway for early screening and clinical monitoring of lipid metabolism-related disorders.
Spontaneous Brillouin scattering detection system based on a dual-stage virtual imaging phase array (VIPA) spectrometer is proposed for elastic imaging of lens capsule and lens section of ex-vivo porcine eye. Results show that the Brillouin longitudinal elastic modulus of the porcine eye lens capsule is approximately 2.90 GPa at the equator and 2.84 GPa at the detect edge, and shows a gradual decrease from the equator toward the posterior pole. The Brillouin longitudinal elastic modulus of the porcine eye lens nucleus is about 3.72 GPa and is distributed in a plateau region at the center of the nucleus, it is about 3.32 GPa at the detect edge, and shows a gradual decrease from the lens nucleus to the surrounding cortical layers. Using Brillouin microscope to characterize the biomechanical properties of porcine eye lens, which is not only important for understanding the process of human eye regulation, but also can provide technical support for the diagnosis and treatment of human hyperopia.
This research proposes a deep learning-based resolution enhancement strategy for near-infrared II (NIR-II, 900?1880 nm) fluorescence imaging, which effectively overcomes the physical limitations of pixel quantity and size of InGaAs and HgCdTe detectors through fine-tuning the Real-ESRGAN super-resolution network model. The effectiveness of this method is validated in three typical scenarios: NIR-II whole-body vascular imaging in mice, multi-scene imaging in the NIR-IIc band, and NIR-II clinical application in diabetic foot assessment. The fine-tuned model significantly outperformed traditional bilinear and bicubic interpolation methods in both perception-based image quality evaluator (PIQE) and spatial resolution indicator—full width at half maximum (FWHM). Notably, the model demonstrated its capability to enhance the resolution of images with similar visual features, even when tested on untrained data from the NIR-IIc band, such as images of the mouse abdomens, intestines, and legs. This technology provides a new solution for high-quality NIR-II biomedical imaging, particularly offering clearer vascular visualization tools for clinical diagnosis, which is expected to play an important role in NIR-II fluorescence-guided clinical surgery.
Fluorescence resonance energy transfer (FRET) serves as a versatile technique for quantifying intermolecular interactions by measuring the energy transfer between donor and acceptor molecules. In this work, a free-addressing FRET technique based on fluorescence lifetime measurements is proposed. Within the framework of a dual-color confocal microscopy, this method utilizes two galvanometric scanners to freely address a focused light to any locations on a sample and uses a time-correlated single photon counting board to capture the trigger signals from the scanners, and the arrival times of photons relative to previous laser pulses. Consequently, the fluorescence lifetime at any region of interest within the field-of-view can be obtained. Once the fluorescent lifetimes of donor fluorophores before and after bleaching the acceptor fluorophores are determined, the FRET efficiency at the targeted site can be further calculated. Compared with the traditional intensity-based FRET measurement method, this approach does not require special calibration operations and significantly reduces measurement times from minutes to milliseconds. We can envisage that the proposed technique can be widely applied to life science, medical diagnosis, materials science, and so on.
Traditional optical property measurement techniques typically rely on complex detection systems and are easily affected by environmental disturbances. To solve these problems, this paper proposes a low?cost and disturbance?resistant single?image inversion method for two?layer dental optical properties, achieved by integrating physical priors with a deep learning fusion framework. By modeling the optical transmission process within the tooth structure, the optical properties of teeth inversion network (OPTNet) is proposed, which preserves multi?layer features through residual concatenation and incorporates a spatial attention mechanism to focus on critical diffuse reflectance regions, thereby enabling precise prediction from a single image. Natural teeth are collected and corresponding restorative samples are fabricated, an optical acquisition system is then assembled to capture the surface light distributions of these samples, thereby constructing the clinical reflectance dataset. On clean clinical samples, the proposed method achieves an average relative error (MRE) below 0.2% and a coefficient of determination (R2) above 0.99. To evaluate robustness, tests are also conducted on samples with measurement noises. Results show that, OPTNet reduces the MRE of the reduced scattering coefficient and absorption coefficient by 55.08% and 72.38% compared to a fully connected network (FCN), respectively, and by 43.01% and 46.77% compared to a traditional convolutional neural network (CNN), respectively. The proposed physics-prior-driven deep learning approach for biophysical measurement achieves high-precision inversion of dental optical properties without requiring complex optical instrumentation, offering a novel solution for tooth color matching and customized restorative treatments.
Impulsive stimulated Brillouin scattering (ISBS) spectroscopy is a recently developed non-contact optical measurement technique widely used for studying the viscoelastic properties of biological tissues and materials. To improve spectral extraction accuracy and sensitivity under low signal-to-noise ratio (SNR) conditions, this study proposes the application of dynamic mode decomposition (DMD) for analyzing ISBS time-domain signals. The DMD method constructs a Hankel matrix from the time-domain signal and extracts its dominant modal features, enabling precise extraction of the Brillouin frequency shift. By simulating various models including single-peak, closely spaced dual-peak, and weak-signal components, this study systematically compares the performance of DMD with the adaptive noise-suppression matrix pencil (ANMP) method under different SNRs. The results demonstrate that the DMD method outperforms ANMP in frequency estimation accuracy, spectral resolution, and weak-component sensitivity, making it particularly suitable for Brillouin signal analysis in complex environments. This approach provides a more stable and efficient spectral analysis tool for ISBS measurement systems under low-SNR conditions and for multicomponent material characterization.
Two-photon light sheet microscopy is widely used in biomedical related fields due to its advantages of high spatio-temporal resolution and low phototoxicity and photobleaching. However, due to the scattering and absorption of excitation light and fluorescence by biological samples, the quality of deep imaging of samples is poor, and it is difficult to realize large-sample, high-throughput imaging due to the narrow sample operating space of traditional light sheet microscopy. To address the above problems, we optimized the spatial layout of the light sheet microscopy system and combined it with a near-infrared II excitation light source, and at the same time, we used an axicon to modulate the ordinary Gaussian illumination beam into a Bessel beam, which achieved a wide range of thin-sheet illumination by virtue of its non-diffractive properties, and finally constructed an open-top two-photon light sheet imaging system based on non-diffractive Bessel beam. The lateral and axial resolutions of the system are (1.85 ± 0.09) μm and (3.23 ± 0.32) μm, respectively, and the field of view is greater than 600 μm, achieving three-dimensional high-resolution imaging. The experiment completed deep tissue imaging of acute kidney injury mice, obtained a large amount of vascular structural information, which facilitates the revelation of pathological changes in acute kidney injury tissues. The experimental results not only contribute to a better understanding of the pathogenesis of acute kidney injury, but also provide a reference basis for future clinical diagnosis and treatment, validating the potential of open-top near-infrared II Bessel two-photon light-sheet microscopy for three-dimensional pathological evaluation.