Besides biochemical characteristics, mechanical properties (such as elasticity and viscosity) also play a crucial role in determining biological functions. Biomechanics is to study the mechanical changes in biological systems and how they respond to mechanical changes in the environment to help early diagnosis and treatment of diseases. However, despite tremendous progress thanks to mechanical measurement technologies like atomic force microscopy, we are still lacking viable techniques that can achieve non-contact measurements at high spatial resolution.
In 2008, Brillouin microscopy was successfully applied to the field of biomechanics for the first time. As a full-optical method, this technology calculates the viscoelasticity of materials by measuring the frequency and attenuation changes of inelastically scattered light caused by density fluctuations (sound waves). This allows for high spatial resolution imaging of mechanical properties in a non-destructive, label-free, and non-contact way. Recently, it has made contributions in areas such as tumor research, developmental biology, and clinical applications for eye diseases.
To meet the urgent demands of biomedical applications, improving imaging speed has become a focal issue for Brillouin microscopy. However, most common Brillouin microscopes rely on the weak spontaneous scattering process, making their spectral measurements highly susceptible to background light and nearly reaching their speed limit (20 ms per spectrum). Stimulated Brillouin microscopy, using a pump-probe scheme to excite resonant phonons, reduces the spectral measurement time to 5 ms, but the use of continuous light sources barely takes advantage of nonlinear interactions. Additionally, because this frequency-domain method relies on frequency scanning and power changes of probe light to map the spectrum, it requires complex frequency sweeping, modulation, and phase-locked amplification processes. This also demands high stability in the frequency and power of the laser source.
Recently, the research group led by Prof. Yan Li from Tsinghua University, in collaboration with Dr. Hongyuan Zhang from Cleveland Clinic, made important progress in high-speed viscoelastic microscopic imaging. They introduced a scheme with a pulsed pump, continuous probe with heterodyne detection, and Matrix Pencil analysis, to measure and decipher Brillouin spectra in the time domain. By optimizing the signal-to-noise ratio (SNR) and accordingly designing the impulsive stimulated Brillouin scattering (ISBS) microscope, they reduced the spectral measurement time to sub-milliseconds while maintaining high spectral accuracy and resolution, enabling three-dimensional mapping of viscoelastic and other mechanical properties of the sample without any contact or labeling. Relevant research results were recently published in Photonics Research, Volume 12, Issue 4, 2024. The first author is Dr. Jiarui Li from Tsinghua University. [Jiarui Li, Taoran Le, Hongyuan Zhang, Haoyun Wei, Yan Li, "High-speed impulsive stimulated Brillouin microscopy," Photonics Res. 12, 730 (2024)]
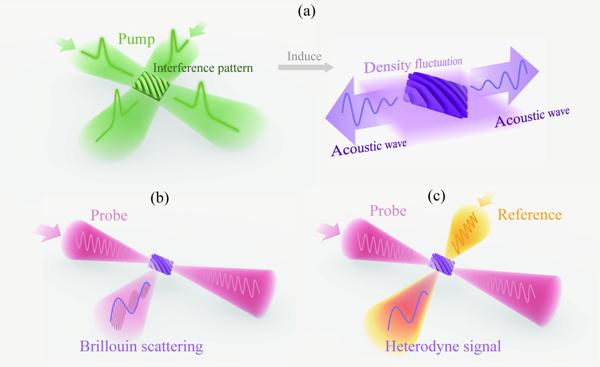
Figure 1 The principle of ISBS with time-domain heterodyne detection. (a) A pair of pulsed pump beams excite acoustic waves in the material. (b) Continuous probe interacts with acoustic waves in the material and results in Brillouin scattering. (c) Another continuous light is introduced as a reference beam for heterodyne detection of the time-domain signal.
In ISBS microscopy, a pair of picosecond pulses pump sound waves in the material [Figure 1(a)]. Due to the broadband characteristics of pulsed laser, there are multiple frequency differences between the two pump lights, allowing resonance with phonon modes of various materials, thus eliminating the need for frequency scanning during measurement. The probe is a narrow-linewidth near-infrared continuous light that interacts with the sound waves in the material, resulting in Brillouin scattering [Figure 1(b)]. The time-domain oscillation of the scattered light, modulated by the sound waves, is measured with a photodetector to obtain sound wave information. Another continuous light, from the same source with a symmetric optical path to the probe light, is introduced for heterodyne detection [Figure 1(c)], enhancing the signal, suppressing common-mode noise, and reducing the system's dependence on the laser source stability.
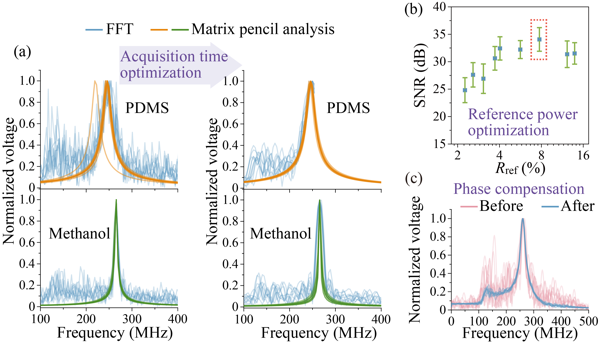
Figure 2 (a) Improvements from Matrix Pencil and optimization of acquisition time, (b) power of reference light, and (c) phase compensation.
For time-domain Brillouin scattering signals, researchers proposed a new method for deciphering Brillouin spectral information, using the Matrix Pencil algorithm instead of the Fourier transform [Figure 2(a)]. This frequency analysis method, which is based on matrix transformation on the time domain, is more compatible with the ISBS signal, which is based on time-domain detection and has only a few frequency components. This method 1) avoids spectral broadening and sidebands caused by reduced acquisition time, thus ensuring spectral resolution; 2) has better noise suppression ability, allowing high spectral accuracy even if the SNR is sacrificed to increase measurement speed or reduce phototoxicity.
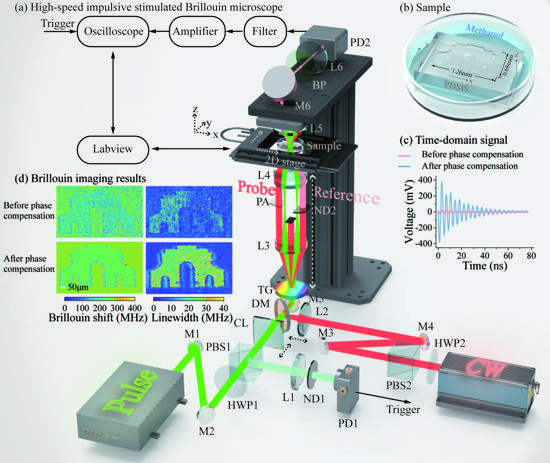
Figure 3 (a) ISBS microscope. (b) Sample schematic. (c) Time-domain Brillouin signal. (d) Imaging results of Brillouin shift (related to elasticity) and linewidth (related to viscosity) before and after phase compensation.
To further increase measurement speed, researchers developed an SNR enhancement solution enabled by an SNR model and corresponding multi-parameter optimization, including acquisition time [Figure 2(a)], reference light power [Figure 2(b)], and phase [Figure 2(c)], and accordingly designed a high-speed ISBS microscope [(Figure 3(a)]. After optimization, the Brillouin shift precision and spectral resolution for methanol remained at 0.7 MHz (0.26% of the frequency shift) and 5 MHz (1.9% of the frequency shift), respectively, with a single-pixel spectral integration time of only 0.3 ms. Using this system, three-dimensional ISBS imaging was further achieved with a common biological material, PDMS, immersed in methanol with a millimeter-scale pattern as the sample [Figure 3(b)]. The imaging results showed spatial variations in multiple mechanical properties, including elasticity, viscosity, and elasto-optic coefficient (Figure 4). Both the sample and solution are transparent and difficult to distinguish with typical bright-field imaging, while, due to the difference in their mechanical characteristics, the microstructure of the sample can be "seen" by the ISBS microscope without any labeling or contact based on the distribution of mechanical features.
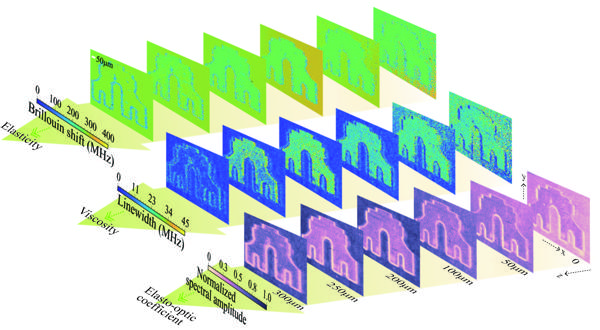
Figure 4 Three-dimensional imaging results from the high-speed ISBS microscope. Mapping results of Brillouin shift, linewidth, and normalized spectral amplitude show spatial variations of elasticity, viscosity, and elasto-optic coefficient, respectively.
This high-speed ISBS microscopy could achieve high spectral accuracy and resolution with sub-millisecond single-pixel integration time. The imaging results showed the distribution of multiple mechanical features including viscoelasticity. In the future, this technology has the potential to capture rapid mechanical changes in biological samples with higher temporal and spatial resolution.







