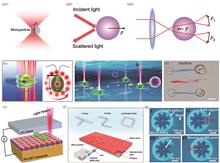
As the central focus in modern life sciences, precision medicine has been significantly propelled by breakthroughs in micro/nano-robotic technology. Compared to other actuation approaches, light-driven micro/nanorobots offer distinct advantages, including non-contact precision manipulation, dynamic programmability, and superior biocompatibility. This paper systematically introduces the key motion-control mechanisms of light-driven micro/nanorobots, including direct optical manipulation, bio-phototactic propulsion, photothermal actuation, photochemical propulsion, and photoinduced-deformation propulsion. It highlights their innovative applications in core areas of precision medicine, such as targeted drug delivery, clearance of biological threats, minimally invasive therapy, disease diagnosis, and biosensing. Current challenges are also critically addressed, encompassing optical control in deep tissues, long-term biosafety in vivo, and intelligent system integration. Future directions are proposed through synergistic integration of advanced optical technologies with artificial intelligence, aiming to advance the intelligent evolution and clinical translation of light-driven micro/nanorobots in precision medicine.
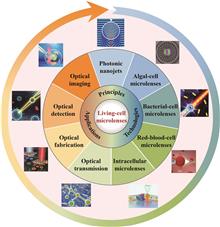
Microlenses have attracted considerable attention in the field of micro-/nano-optics due to their unique optical properties and simple structures, which allow them to be integrated into diverse optical systems to enhance optical performance. However, artificially fabricated optical microlenses face limitations in biocompatibility, biodegradability, and dynamic responsiveness. Natural biological optical microsystems provide inspiration for overcoming these constraints. It has been found that living cells and their microstructures exhibit lensing effects, functioning as natural dynamic optical components capable of performing optical functions within biophotonics research platforms. This paper analyzes biological microlensing techniques associated of algae, bacteria, red blood cells, and subcellular structures, and systematically summarizes their innovative application outcomes in key areas such as optical imaging, optical detection, optical manipulation, and optical transmission. Furthermore, the challenges and prospects for microlenses derived from living cells in the development of biophotonic devices are discussed.
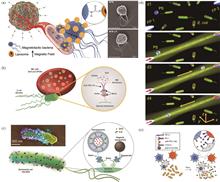
With the synergistic advancement of micro/nano-fabrication technologies and intelligent control systems, medical microrobots are progressively overcoming the dual limitations of operational precision and invasiveness inherent in traditional diagnostic and therapeutic techniques, thereby providing innovative tools for precision medicine in critical diseases. However, current research predominantly focuses on artificially synthesized materials, whose complex fabrication processes and inherent immunogenicity risks not only lead to rapid clearance by the host immune system but also restrict their long-term therapeutic applications in deep tissues. The in situ construction of medical microrobots based on natural biological cells, achieved through precise modulation of their behaviors via external fields, may offer a transformative solution to resolve these challenges. This review systematically summarizes the design strategies of seven representative cell-based microrobots, including chemotactic bacteria, photosynthetic microalgae, autonomous contractile cardiomyocytes, red blood cells, platelets, macrophages, and neutrophils, with a focus on their recent advancements in drug delivery, minimally invasive surgery, and immunotherapy. Furthermore, this work critically analyzes the current challenges while outlining future research directions and clinical translation prospects.