Introduction: From wide-field fluorescence microscopy to super-resolution structured illumination microscopy
Fluorescence microscopy, as a pivotal tool in the life sciences, grapples with the challenges of background fluorescence interference and the optical diffraction limit. Structured Illumination Microscopy (SIM), a significant branch of super-resolution techniques, can enhance resolution up to twice the diffraction limit. It boasts the advantages of being "gentle" (lower excitation intensity, minimizing sample damage) and "inclusive" (no need for special labeling or complex processing), making it a recent focus of research.
Recently, Professor Peng Xi's team at Peking University was invited to author a review article titled "High-spatiotemporal-resolution structured illumination microscopy: principle, instrumentation, and applications", which is featured as the cover article in Issue 1 of Photonics Insights in 2025. This comprehensive review revisits the fundamental principles of SIM, the system implementation based on various high-speed optical modulation devices, the applications of SIM in cutting-edge biological fields, and prospects to the future development directions of SIM.
Basic Principles of SIM
As the name suggests, SIM utilizes illumination patterns with specific spatial distributions to excite sample fluorescence. Essentially, structured light modulates the actual spatial information of the sample.
In a diffraction-limited microscope system, the image of an ideal point source appears blurred as an Airy disk, which is known as the system's point spread function (PSF). The Fourier transform of the PSF gives the optical transfer function (OTF), which has a low-pass characteristic, with a cutoff frequency equal to the inverse of the diffraction limit (Figure 1a, b). SIM works by encoding high-frequency spatial information into lower-frequency moiré fringes (Figure 1c), allowing such information to pass through the optical system and finally be reconstructed.
The simplest structured illumination pattern is a sinusoidal fringe. Under sinusoidal modulation, the observable frequency domain of the system expands, leading to higher spatial resolution (Figure 1d). By rotating the sinusoidal fringes in different directions, an almost isotropic OTF extension can be achieved (Figure 1e).
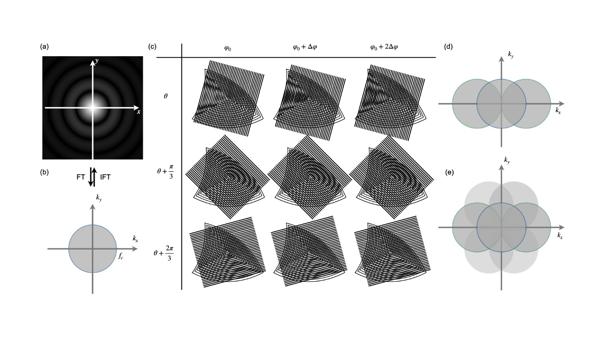
Figure 1 a) The image of an ideal point source; b) The optical transfer function; c) Moiré fringes in different directions and with different phases; d) The expansion in the frequency domain by sinusoidal patterns; e) Isotropic expansion of observable regions in the frequency domain.
The fundamental and most important method for generating uniform structured fringes on a sample surface is light interference. Compared to wide-field fluorescence microscopy (Figure 2a), SIM can produce structured fringes of specific frequencies by directing two coherent beams into the objective's pupil. By adjusting the spacing between the two beams, total internal reflection fluorescence (TIRF) mode can be achieved, restricting the sinusoidal fringes to the sample's shallow surface, thus enhancing axial resolution and contrast (Figure 2c). If three beams interfere simultaneously, structured fringes can also be generated along the axial direction, leading to an axial resolution enhancement (Figure 2d).
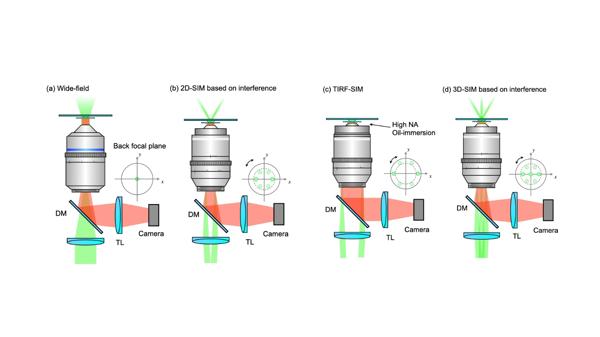
Figure 2 a) Wide-field fluorescence microscopy; b) 2D-SIM; c) TIRF-SIM; d) 3D-SIM.
In addition to fringes, focused light spots (or spot arrays) scanning across the sample plane can also serve as structured illumination. A pair of conjugate pinholes theoretically improves resolution by a factor of ~1.41. Building upon this principle, high-speed imaging and reconstruction have become the main developmental focus of point-scanning SIM techniques.
Implementation and technological advances in SIM
Precise control of the illumination pattern is key to SIM implementation, as its switching speed and accuracy directly affect the spatiotemporal resolution of imaging. Diffraction gratings are the core component of stripe-based SIM techniques, where ±1st-order diffracted beams interfere to generate periodic sinusoidal fringes. Rotation and phase shifting of the structured illumination pattern are achieved by physically rotating and translating the grating, and the imaging speed is limited. For point-scanning SIM techniques, the spatiotemporal resolution is primarily influenced by the number of raw images and the precise stepwise movement of the focused spots.
With the rapid development of high-speed optical modulators such as spatial light modulators (SLMs), digital micromirror devices (DMDs), and galvanometer scanners (Galvos), the spatiotemporal resolution of SIM has been significantly enhanced.
- In stripe-based SIM, SLMs generate pixelated grating patterns, allowing rapid switching of pattern directions and phases, thereby improving imaging speed and resolution. (Figure 3a)
- Galvo-based SIM systems typically employ two fully symmetrical scanning units to precisely control the scanning path and frequency of the laser, enabling high-speed SIM imaging. (Figure 3b)
- DMDs, as a novel high-speed optical modulator, have a high switching frequency and can operate stably while continuously refreshing patterns. The low cost and high efficiency have led to their increasing application in SIM. (Figure 3c-d)
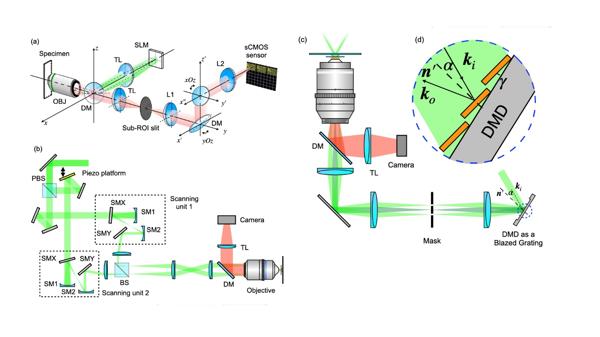
Figure 3 Stripe-based SIM implementation. a) SIM based on SLM; b) SIM based on galvos; c-d) SIM based on DMD.
In point-scanning SIM, precise and rapid laser scanning control is essential. Common devices include DMDs, galvanometers, and spinning disks.
- DMDs are used to generate sparse two-dimensional excitation patterns, enabling efficient imaging through stepwise scanning (Figure 4a). Combined with digital localization and deconvolution techniques, they can effectively improve imaging speed and reduce data volume.
- Galvanometer scanners enable fluorescence emission rescanning, redistributing photon paths and reducing data processing demands (Figure 4b).
- Spinning disks, with their high-speed scanning capabilities and integration with microlens arrays, enhance light efficiency while improving temporal resolution (Figure 4c).
The application of high-speed optical modulators has significantly boosted SIM's spatiotemporal resolution, driving the advancement of structured illumination microscopy.
.png)
Figure 4 Point-scanning SIM Implementation. a) System based on DMD; b) System based on galvos; c) System based on spinning disks.
Applications and Breakthroughs of SIM in Biological Research
Compared to conventional fluorescence microscopy, SIM surpasses the diffraction limit by achieving twice the resolution enhancement. With its high spatiotemporal resolution and low phototoxicity, SIM has become one of the most popular SRM techniques in biological research.
SIM significantly improves the clarity of biological structure imaging. Researchers have used SIM to observe interactions between cells and viruses, providing crucial insights into viral pathogenesis (Figure 5a). Additionally, nonlinear SIM has successfully resolved the ring-like structure of nuclear pore complexes, as well as intricate cytoskeletal structures such as F-actin (Figure 5b).
SIM is particularly suitable for live-cell imaging. It has been applied to studying dynamic changes in mitochondria, including fission and fusion processes (Figure 5c). Even more complex phenomena, such as the contraction of endoplasmic reticulum tubules, have been captured with clarity, providing a powerful tool for cell dynamics research (Figure 5d).
.jpeg)
Figure 5 SIM reveals subcellular structures and dynamic processes with high spatiotemporal resolution. a) Precise PML-NB organization in JC virus-infected human glial cells detected by SIM. b) The formation of an F-actin nanoscale ring. c) PAR-SIM captures mitochondrial kiss-and-run and extrusion events, with time-encoded pseudo-color trajectories. d) ER tubule dynamics.
The multicolor imaging capability of SIM enables the study of organelle interactions. Multicolor SIM has revealed interactions between mitochondria and lysosomes (Figure 6a), mitochondria and microtubules, mitochondria and the endoplasmic reticulum, as well as interactions between microtubules and actin, deepening our understanding of cellular regulation.
For 3D imaging, SIM has demonstrated outstanding capabilities. For instance, MC-ISM has successfully achieved 3D imaging of plant cell mitochondria, highlighting similarities between plant and animal mitochondria (Figure 6b). By combining adaptive optics with multifocal SIM, researchers have achieved deep tissue imaging, revealing key processes such as axonal extension and synapse formation during neural development (Figure 6c).
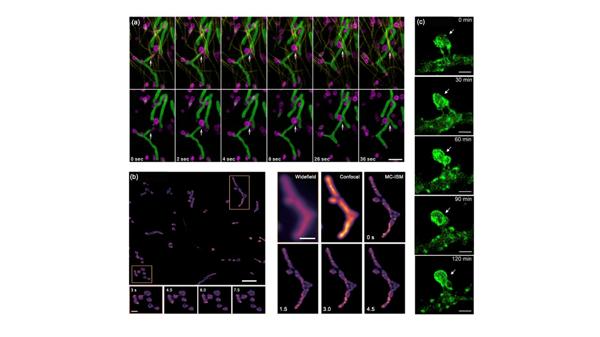
Figure 6 Multicolor imaging and observation of deeper biological phenomena with enhanced imaging depth. a) Interactions between mitochondria and lysosomes. b) Arabidopsis hypocotyl mitochondria. c) Dynamic morphological changes in a specific axonal protrusion.
Conclusion and Outlook
In recent years, SIM has become increasingly important in biomedical research, serving as a crucial tool for studying cellular functions and structures. This review evaluates various performance aspects of classical SIM imaging systems and presents radar charts to help researchers select the most suitable technique (Figure 7).
With the growing demand for high-resolution imaging, SIM is being integrated with other microscopy techniques to enhance its capabilities. Combining SIM with electron microscopy and quantitative Förster resonance energy transfer (FRET) expands its imaging potential. Additionally, integrating SIM with label-free imaging, such as 3D optical diffraction tomography, provides deeper insights into live-cell imaging while reducing phototoxicity.
Looking ahead, advancements in SIM will include multi-plane parallel imaging, polarized SIM, and other emerging techniques. These innovations will expand SIM's applications in life sciences, driving further discoveries in cellular mechanisms and disease research. As biological exploration continues, SIM is poised to evolve into a more powerful super-resolution imaging tool, unlocking deeper insights into subcellular structures and dynamic processes.
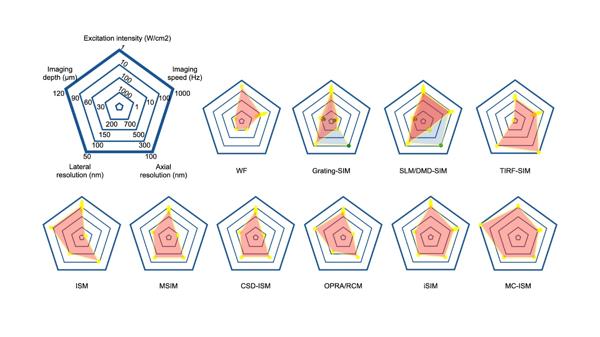
Figure 7 Summary and performance comparison of SIM techniques in radar maps. The light blue area and green points stand for performances in 3D-imaging mode.







