.jpeg)
1. Research Background
Biological specimens and industrial microstructures are often transparent or translucent. Traditional microscopy faces challenges in observing such phase objects, including low contrast and the inability to directly obtain quantitative information. Quantitative phase imaging (QPI), particularly Fourier ptychographic microscopy (FPM)-based QPI (FPM-QPI), has rapidly emerged as a powerful label-free microscopy technique. FPM-QPI intelligently integrates concepts of synthetic aperture imaging and phase retrieval, overcoming the inherent trade-off between field of view (FOV) and spatial resolution in conventional microscopy. It enables simultaneous wide-FOV and high-resolution quantitative phase measurements. Phase information directly reflects the optical path length delays introduced by samples, allowing precise characterization of cellular properties (e.g., dry mass distribution, growth dynamics) and industrial sample topography (e.g., semiconductor wafers, glass surfaces). Over the past 12 years, continuous advances in FPM hardware and algorithms have significantly expanded its applications in biomedicine and industrial inspection.
Despite its growing advances, no prior review has systematically addressed the interdisciplinary progress of FPM-QPI. Existing literature focuses on isolated technical aspects or specific applications, leaving a critical gap in understanding the holistic evolution of this field.
Recently, the research teams led by Prof. Qun Hao and Assoc. Prof. Shaohui Zhang at Beijing Institute of Technology, in collaboration with Prof. Fei Liu and Assoc. Prof. Meng Xiang at Xidian University, published a review titled"Quantitative phase imaging based on Fourier ptychographic microscopy: advances, applications and perspectives" in Advanced Imaging, and was selected as cover paper for 2025, Vol. 2, Iss. 3. This review systematically outlines the development of FPM-QPI, starting from its fundamental principles. It highlights key advances in three main branches (Fig. 1): phase retrieval algorithms, intensity diffraction tomography (IDT), and artificial intelligence (AI) and details representative applications in label-free live-cell monitoring, digital pathology, clinical diagnosis, and surface topography, and discusses current challenges and future trends.
.jpeg)
Figure 1. The three main development branches of FPM-QPI: phase retrieval algorithms, intensity diffraction tomography, and artificial intelligence
2. Research Progress
(1) Fundamental principles of FPM-QPI
FPM-QPI is a computational imaging technique that overcomes traditional microscopy's trade-off between FOV and resolution. It replaces a microscope's condenser with a programmable LED array to illuminate samples from varying angles. Each activated LED generates a plane wave, and the corresponding low-resolution (LR) intensity image captured by the sensor contains unique spatial frequency information. Critically, darkfield LEDs (positioned beyond the objective's numerical aperture) encode high-frequency details. The core innovation lies in computationally synthesizing hundreds of these LR images through an iterative phase retrieval process.
By modeling light-sample interactions and enforcing physical constraints (e.g., amplitude matching to measurements and pupil function constraints), FPM-QPI reconstructs a high-resolution complex field—simultaneously recovering amplitude and quantitative phase across a wide FOV. This effectively synthesizes a larger numerical aperture ("synthetic aperture"), achieving resolution beyond the optical limits of the objective lens. Unlike interferometric methods, FPM-QPI operates without coherent reference beams, using partially coherent LED illumination to minimize speckle noise. Its label-free phase quantification enables nanoscale sensitivity to optical path differences, revealing intrinsic sample properties like cellular dry mass or surface topography. Advancements in algorithms (e.g., joint pupil aberration correction) and AI integration further enhance its robustness, speed, and applicability to thick samples via 3D refractive index tomography.
(2) Applications of FPM-QPI
- Label-Free Live-Cell Monitoring and Analysis. FPM-QPI enables long-term, high-resolution observation of subcellular structures and dynamics (e.g., cell division, lipid droplet transport) without staining, as shown in Figure 2.
.jpeg)
Figure 2. Label-free cell monitoring and analysis with FPM-QPI
- Digital Pathology and Clinical Diagnosis. FPM-QPI provides novel quantitative phase information for pathological slides. Compared with conventional microscopy, the phase images recovered by FPM provide a completely new dimension of information for pathologists. Mining the benefits of phase information for digital pathology and clinical diagnosis is a long-standing research topic, stimulating extensive research shown in Figure 3.
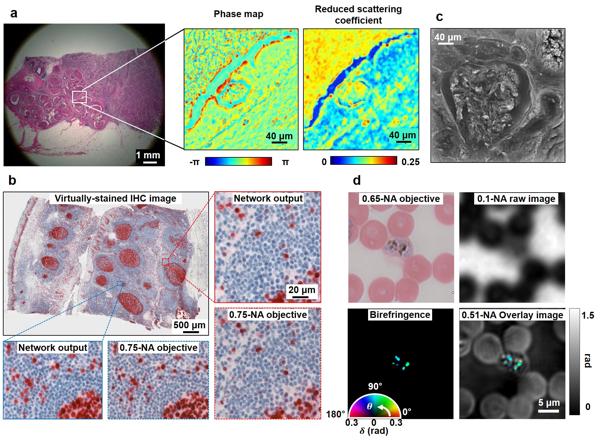
Figure 3. Digital pathology and clinical diagnosis with FPM-QPI
- Surface metrology. FPM-QPI enables large-area, non-contact, high-resolution surface metrology for industrial inspection, including defect detection, high-energy and microstructure characterization.
3. Outlook
(1) Current Challenges
- Improving Phase Transfer Function (PTF): Non-uniform PTF under non-ideal illumination compromises low-frequency phase recovery accuracy. Simpler methods for achieving ideal PTF are needed.
- Calibrating Physical Model Mismatches: Deviations from idealized assumptions (plane-wave illumination, thin sample, aberration-free optics) degrade reconstruction. Computationally efficient non-convex phase retrieval algorithms are essential.
(2) Research Trends
- High-Speed and Long-Term Imaging: Integrating high-speed programmable illumination with deep-learning reconstruction can accelerate acquisition, reduce redundancy, and enhance noise robustness. Tighter hardware-software integration is needed to counter environmental instability.
- Multi-Modal Fusion: Multispectral or hyperspectral FPM can probe RI dispersion. Combining fluorescence or Raman imaging enriches functional insights while preserving label-free QPI.
- 3D QPI: Advancing forward models and reconstruction algorithms (especially AI-driven) for IDT will improve accuracy, speed, and robustness in revealing complete 3D structures.
(3) Application Trends
- Life Sciences: Growing demand in cell and gene therapy (CGT) for label-free, real-time cell monitoring (viability, density). FPM-QPI's high space-bandwidth product (SBP) could replace fluorescent labeling.
- Clinical Diagnosis: Deployment requires high throughput and cost-effectiveness. Drug screening and tissue diagnosis (e.g., phase-derived fractal biomarkers) show promise, but a deeper understanding of tissue optical properties (e.g., RI variability) is needed.
- Surface Metrology: Hybrid interferometric-FPM architectures with spectrally engineered illumination may decouple sensitivity from mechanical scanning, enabling simultaneous ultrahigh axial resolution (<λ/100) and kHz acquisition rates.







