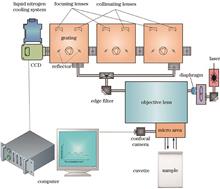
To improve the detection performance of glucose solutions, we first simulate and calculate the theoretical Raman spectrum of 99% (volume fraction) glucopyranose in the glucose solution. Next, we obtain experimental Raman spectra of glucose solutions at different mass concentrations using a Raman spectroscopy system. The theoretical calculations show that both types of glucopyranose exhibit characteristic peaks at 1125 cm-1, which is consistent with the experimental results. We then select the 1640 cm-1 characteristic peak of water as the internal standard peak for normalization. Finally, two quantitative analysis methods, the characteristic peak relative intensity method and the characteristic peak relative area method, are used for linear analysis. The linear correlation coefficient are 0.992 and 0.985, respectively. The lowest detection limits are 48.1 mg/dL and 52.3 mg/dL, and the lowest detection mass concentrations are all 12.5 mg/dL. The results indicate that the relative intensity method is simple and convenient, and provides a higher linear fitting, making it more suitable for accurate quantitative analysis of low mass concentration glucose solutions.
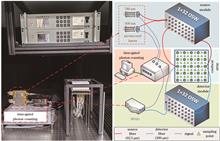
We introduce a confocal functional near-infrared spectroscopy (fNIRS) imaging system that utilizes a time-gated photon-counting technology. This innovative approach enables the acquisition of high sensitivity and high spatial resolution information within a confocal array while considering cost-effectiveness. The performance of the system is confirmed through a series of functional tests and phantom experiments. Results from these tests show that the system can freely set the position and width of the time gating window within a range of 0 to 10 ns, with a temporal resolution of 10 ps. Phantom experiment results indicate that the system achieves a quantitative ratio improvement of over 32.9% in the confocal array, an enhancement of more than 31.6% in the contrast-to-noise ratio, and an increase of over 29.5% in spatial fidelity. Therefore, the confocal fNIRS imaging system designed in this study, using time-gated photon-counting technology, effectively improves imaging quality at a reasonable cost. This provides instrumental support and methodological reference for related fNIRS research.
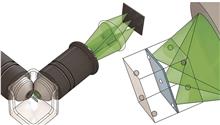
Fluorescence microscopy is a vital tool in biomedical research, enabling high-resolution imaging by using fluorescent dyes or proteins to label specific cells or molecules, which then emit fluorescence under a microscope. Light-sheet fluorescence microscopy (LSFM) is an emerging three-dimensional imaging technology that achieves high-throughput, high-resolution 3D imaging by rapidly scanning thin samples. This technique offers advantages such as low phototoxicity and photobleaching, high photon efficiency, fast imaging speed, and high resolution. It is widely used in fields such as neuroscience, cell biology, and pathology. LSFM shows great potential in three-dimensional pathological analysis. Unlike traditional two-dimensional pathology slides, 3D pathological analysis provides complete spatial information on tissue structures, aiding in a more comprehensive understanding of disease mechanisms. The development of 3D pathological analysis significantly advances pathological research and clinical diagnostics, offering strong support for early disease detection, precise treatment, and personalized medicine. We first introduce the development of light-sheet microscopy and its applications in the pathological field, then discuss the main current approaches and methodologies for 3D pathological analysis. We focus on the potential applications of emerging multimodal large language models in pathological analysis.