Abstract
Copper-based organic light-emitting diodes (OLEDs) are low-cost alternatives to precious metal-based OLEDs, but currently no such OLEDs can meet the practical requirements for high colour purity, device efficiency, and operational stability. Carbene-Cu(I)-amide emitters reported here exhibited thermally activated delayed fluorescent emission with quantum efficiencies up to 0.90 and radiative decay rates of 2.7 × 106 s−1. These enable blue to near-infrared Cu(I)-OLEDs with high brightness (265,000 cd m−2) and extended LT95 lifetime (3582 hours at 1000 cd m−2). Deuteriation and π-extension of carbazole significantly enhance OLED stability. Cu(I)-sensitized fluorescence OLEDs showed efficient narrowband electroluminescence (λmax 612–614 nm; full-width half maximum of 33–38 nm; maximum external quantum efficiencies reach 21.9%) and prolonged LT95 lifetime (up to 3689 h at 1000 cd m−2). This work highlights earth-abundant metal-based sensitized-OLEDs that exhibit high colour purity and long device lifetime comparable to the best non-iridium metal-based OLEDs.
Introduction
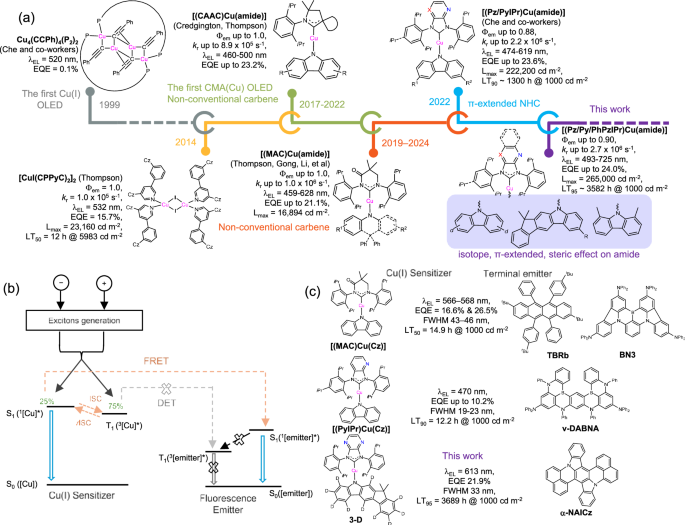
Metal emitters/sensitizers are indispensable in current organic light-emitting diode (OLED) technology as metal atoms play an integral role in promoting intersystem crossing/reverse intersystem crossing (ISC/rISC) and radiative decay of triplet excited states, but they are mainly developed from low earth-abundance noble metals1,2,3,4,5. Therefore, in order to achieve sustainable development and reduce the manufacturing cost of OLEDs, there is growing interest in developing metal emitters/sensitizers utilizing earth-abundant metals. In this regard, copper(I) thermally activated delayed fluorescence (TADF) emitters are an attractive low-cost alternative to precious metal emitters such as iridium(III) or platinum(II) emitters in OLED applications. Cu(I)-TADF emitters have been intensely studied over the past decades and exhibit high emission quantum yields (Φem), providing high external quantum efficiency (EQEs) for OLEDs6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22. However, despite decades of research, reported Cu(I)-OLEDs still lag far behind threshold requirements for industrial applications, including high color purity, device efficiency, and operational stability.
From our perspective, molecular engineering of two-coordinate carbene-copper(I)-amide (CMA(Cu)) emitters appears to be a promising approach to develop Cu(I) emitter for practical OLED applications10,11,17,18,19,20,21,22,23. For example, by using bulky π-extended N-heterocyclic carbene (NHC) acceptor ligands (i.e., pyridine- or pyrazine-fused PyIPr/PzIPr NHCs) and carbazole donor ligands (Fig. 1a)22,23, CMA(Cu) emitters exhibit efficient 1[Cz→carbene] ligand-to-ligand charge transfer (1LL’CT) emission with Φem of near unity and emission decay lifetimes (τem) of sub-microseconds. This results in a radiative decay rate constant (kr) as high as 2.2 × 106 s−1. OLEDs using these CMA(Cu) emitters have achieved high brightness (up to 2.2 × 105 cd m−2) and significantly improved operational lifetimes compared to literature values for other Cu(I) emitters (Fig. 1a)22. While the improvement is significant, the optimal LT90 value is only achieved at 1300 h at an initial luminance of 1000 cd m−2, which is still far behind the requirements for commercial practical applications24,25. Since the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO) of CMA(Cu) emitters are mainly located on the carbene and carbazole ligands, respectively, structural modification of these two ligands can improve the photophysical properties and stability of CMA(Cu) emitter, thereby enhancing OLED performance and operational stability (Fig. 1a). Although there are many studies on improving the performance and lifetime of OLED devices by modifying carbene ligands, there are few related studies on carbazole ligands. We envisioned developing more efficient and stable CMA(Cu) emitters by structurally modifying carbazole ligands and combining them with π-extended NHC ligands. We considered the following aspects: (i) deuterium atom labeling to introduce stronger C-D bonds and smaller vibrational coupling between the excited and ground states, (ii) increased steric hindrance to restrict conformational flexibility, and (iii) introduction of π-extension to achieve larger HOMO-LUMO separation. Such deuteration, steric, and π-extension effects of carbazole ligands are expected to improve the stability and TADF properties of CMA(Cu) emitters and affect the stability of excitons generated during OLED operation26,27,28,29,30,31.
Fig. 1: Selected examples of Cu(I)-based (TSF/TST-)OLEDs.
a Representative examples of Cu(I)-OLEDs and their performance during 25 years’ development; Φem, kr, λEL, EQE, Lmax, LTx refer to the emission quantum yield, radiative decay rate constant, electroluminescence peak maxima, external quantum efficiencies, maximum luminance, and operational lifetime at x% of initial luminance, respectively. b Schematic mechanism of TSF-OLEDs; S0, S1 and T1 refer to the ground state, singlet excited state, and triplet excited state, respectively, of the Cu(I) sensitizer (denoted as [Cu]) or the fluorescence emitter (denoted as [emitter]). c Examples of TSF/TST-OLEDs using CMA(Cu) sensitizers and organic fluorescence or TADF emitters.
CMA(Cu) complexes belong to donor-acceptor type metal-TADF materials, which usually have a broad emission band, resulting in poor color purity. This is due to their LL’CT properties leading to structural shifts between the ground and singlet excited states32. This problem can be ameliorated by adopting a metal-TADF-sensitized fluorescent/TADF (TSF/TST) strategy, in which a metal-TADF emitter is used as a sensitizer to harvest singlet and triplet excitons and transfer these excitons (through the Förster energy transfer (FRET) process) to organic fluorescent or TADF compounds that have narrower bandwidth emission (Fig. 1b)22,23,33,34,35,36. Compared with organic-TADF emitters, CMA(Cu) emitter is a good TSF sensitizer because of its significantly higher rISC rate constant (krISC; ~107–108 vs. ~105–106 s−1)32. The improved krISC helps to suppress the competing Dexter energy transfer (DET) process, thereby reducing the non-radiative decay energy loss and triplet exciton lifetime in TST-OLEDs (Fig. 1b). It was reported that TSF/TST-OLEDs containing (MAC*)Cu(Cz) sensitizer and TBRb and BN3 emitters showed yellow electroluminescence with full-width-half-maximum (FWHM) as narrow as 30 nm and 43–46 nm, respectively (Fig. 1c)36. The TST-OLED using (PyIPr)Cu(Cz) sensitizer and ν-DABNA emitter showed blue electroluminescence with a narrower FWHM of 19 nm (Fig. 1c)22. However, these reported Cu-sensitized TSF/TST-OLEDs are only at the proof-of-concept stage due to the short device operational lifetime (LT90 or LT50 of ~10 h at 1000 cd m−2; Fig. 1c)22,36. Currently, mainstream sensitizers are still based on luminescent precious metal complexes (i.e., Ir and Pt), and the development of Cu-TADF sensitizers for practical TSF/TST OLED applications is hampered by their stability in OLED device operation.
In the present work, we utilized modified carbazole donor ligands 1,3-dihydro-3,3-dimethylindeno[2,1-b]carbazole (DMIC) for 3-H, perdeuterated carbazole for 1-D, 2-D, 3-D, 6tBu-D and 7-D, 1,8-dimethylcarbazole for 4, or a quinoxaline-fused NHC acceptor for 5 and 6 to prepare CMA(Cu) complexes. Complexes 1–7 show blue to deep-red TADF emission (470–677 nm) in thin films with medium-to-high Φem of 0.39–0.90, short τem of below 1.2 µs, and large kr of 1.2–2.7 × 106 s−1. OLEDs based on these complexes as emitters exhibit EL λmax of 488–722 nm and EQEmax as high as 24.0%, with efficiency roll-off as small as 0.6% at 1000 cd m−2 and brightness up to 265,000 cd m−2. Notably, the operating lifetime (LT95) of these Cu-OLEDs can reach 3582 hours at an initial luminance (L0) of 1000 cd m−2. This LT95 value is one of the few best values reported in the public domain for all organic TADF and metal-TADF emitters. Furthermore, efficient and robust TSF-OLEDs using Cu(I) sensitizer and α-NAICZ as fluorescent emitter achieved narrowband EL λmax 612–614 nm (FWHM of 33–38 nm), high EQE (reaching 21.9%), brightness up to 162,000 cd m−2, and LT95 up to 3689 h at 1000 cd m−2.
Results and Discussion
Synthesis and characterization
Complexes 1–7 with modified carbazole donor ligands (1,3-dihydro-3,3-dimethylindeno[2,1-b]carbazole (DMIC) for 3-H; perdeuterated carbazole for 1-D, 2-D, 3-D, 6tBu-D and 7-D; 1,8-dimethylcarbazole for 4) and quinoxaline-fused N-heterocyclic carbene (NHC) acceptor ligands (5–6) (Fig. 2a) were synthesized according to the reported method (Supplementary Fig. 1)22,23. These complexes were characterized by nuclear magnetic resonance (NMR), high-resolution mass spectrometry (HR-MS), single-crystal X-ray diffraction (XRD), and thermal gravimetric analysis (TGA) (Supplementary Methods; Supplementary Discussion; Supplementary Fig. 2–5, 36–55; and Supplementary Table 1–3). Among them, the 1-H, 2-H and 7-H complexes have been reported in our previous work22. Their electrochemical properties were studied using cyclic voltammetry (CV) and differential pulse voltammetry (DPV) (Supplementary Fig. 6–7; and Supplementary Table 4).
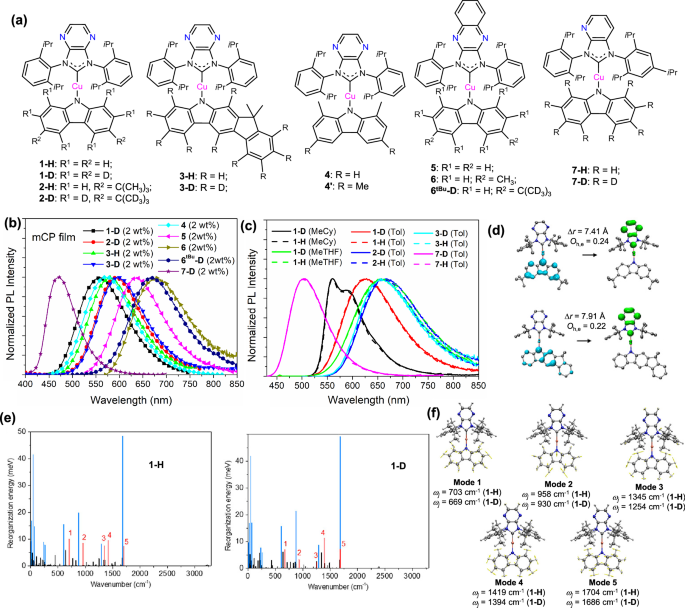
Fig. 2: Chemical structures, photophysical measurements and theoretical calculations of CMA(Cu) emitters 1–7.
a Chemical structures of CMA(Cu) emitters in this work. b Emission spectra of emitters 1–7 doped in 1,3-Bis(N-carbazolyl)benzene (mCP) thin film (in a concentration of 2 wt%) by drop-cast method. c Emission spectra of 1-D, 2-D, 3-D, 7-D (solid line) and 1-H, 2-H, 3-H, 7-H (dashed line) measured in degassed methylcyclohexane (MeCy), toluene (Tol), or 2-methyltetrahydrofuran (MeTHF). Emission spectra of 1-H, 2-H and 7-H have been reported previously22. d Calculated hole and electron distribution, the overlap between hole and electron wavefunctions (Oh,e) and the distance between centroids of hole and electron (Δr) of coplanar structures of 2-H and 3-H in the S1 excited state; blue and green isosurfaces represent hole and electron distributions, respectively. e Reorganization energy distribution of S1 → S0 vibrational relaxation for 1-H and 1-D, red lines represent major vibrational modes localized on the carbazole ligand, blue lines represent other major vibrational modes, and black lines represent minor vibrational modes; f Major vibrational modes 1-5 localized on the carbazole ligand, the corresponding vibrational frequencies (ωj) for 1-H and 1-D are labeled.
Photophysical properties
The photophysical properties of 1–7 were studied (Fig. 2b, c; and Supplementary Fig. 8–12; Supplementary Table 5). Preliminarily, 2 wt% Cu(I) emitters were doped in 1,3-bis(N-carbazolyl)benzene (mCP) thin films via drop-cast (DC) method. These thin films showed blue to deep red emission with peak maximum (λmax) ranging from 470 to 677 nm, emission decay lifetime (τem) below 0.46 µs, and medium-to-high emission quantum yields (Φem) of 0.39–0.75 (Fig. 2b; Supplementary Table 5). These Cu(I)-doped mCP thin films exhibit large radiative decay rate constants (kr) in the range of 1.2–1.9 × 106 s−1, which are comparable to previously reported CMA(Cu) emitters with π-extended NHC ligands and are among the highest Cu(I) TADF-emitters reported to date10,11,22.
The deuterium isotope effect on the photophysical properties of these Cu(I) emitters is then explored. In degassed toluene, the emission λmax of deuterated emitters 1-D, 2-D, 3-D, and 7-D are consistent with those of corresponding non-deuterated emitters (Fig. 2c)22. Notably, in toluene, red-emitting 1-D, 2-D, and 3-D have higher Φem (increased by 29–100%) and smaller non-radiative decay rate constants (knr; 1.3–3.5 folds of decrease) compared to their non-deuterated analogs, whereas their kr values are comparable (Supplementary Table 5)22. In contrast, 7-D and 7-H exhibited green-emission with similar Φem, kr and knr values in toluene (Supplementary Table 5)22. These observations suggest that deuterium substitution in the carbazole ligand does not affect the singlet ligand-to-ligand charge transfer (1LLCT) energy but leads to a decrease in the knr of the red-emitting compounds. Variable temperature lifetime measurements reveal that the singlet-triplet energy gap (ΔEST) of 1-D is 51 meV and the singlet state decay time (τs1) is 17 ns (Supplementary Fig. 10a). According to the equation kS1 = Φem/τS1, the S1→S0 radiative rate constant (kS1) for 1-D is 3.5 × 107 s−1. These TADF parameters for 1-D are very close to those of 1-H (ΔEST of 50 meV, kS1 of 3.5 × 107 s−1)22. The fs-TA spectra of 1-D and 2-D recorded in toluene show the same spectral changes as the non-deuterated analogs with comparable inter-system crossing (ISC) rate constants (kISC; 4.5–5.0 × 109 s−1 for 1, 2.2–2.3 × 109 s−1 for 2; Supplementary Fig. 12). These results indicate that the ISC process of these CMA(Cu) emitters is not affected by the deuterium isotope effect, which is consistent with the observations of other reported deuterium-labeled TADF emitters27,28,29,30.
Theoretical calculations
To gain deeper insights, density functional theory (DFT) and time-dependent density functional theory (TD-DFT) calculations were performed. The optimized 3-H and 4’ ground state (S0) geometries are in good agreement with the experimentally obtained crystal structures (Supplementary Table 6). Geometry optimization of the 1-H, 2-H, and 3-H complexes in the S1 and T1 excited states resulted in two structures with coplanar or orthogonal orientations between the carbazole and carbene ligands (Supplementary Fig. 13). This shows that 1-H, 2-H and 3-H can undergo flexible dihedral angle rotations in the excited states. However, geometry optimization of the 4’ excited state resulted in only confined structures with torsional angles (θN1-C1-N2-C2) of 30–40° due to inter-ligand repulsion between bulky substituents on the ligands (θN1-C1-N2-C2 = 41° for S1 state and 31° for T1 state).
The frontier molecular orbital diagrams of 3-H and 4’ are shown in Supplementary Fig. 14. Their HOMO and LUMO are localized on the carbazole and carbene ligands, respectively. The excitation of electrons from HOMO to LUMO results in the S0 → S1 1LLCT transition. To further elucidate the excited state characteristics, we calculate the hole (h+) and electron (e−) distribution diagrams of 2-H and 3-H in the S1 excited state (Fig. 2d and Supplementary Fig. 15)37,38. Compared with 2-H with a tert-butyl substituent on the carbazole ligand, the π-extended fluorene substituent on the carbazole ligand leads to a longer distance and less overlap between the hole and electron of 3-H (Fig. 2d). The calculated ΔEST value of 3-H is about 0.03 eV smaller than that of 2-H (in coplanar geometry, ΔEST = 0.10 eV for 3-H and 0.13 eV for 2-H). The narrower ΔEST would promote the fast rISC process of 3-H, leading to a higher kr value and more efficient TADF of 3-H compared with 2-H (in toluene, kr = 2.3 × 106 s−1 for 3-H and 1.3 × 106 s−1 for 2-H; Supplementary Table 5)22.
To investigate the effect of ligand deuteration, we performed vibrational mode analysis of 1-H and 1-D. The total reorganization energy is projected into the vibrational relaxation from the S1 state to the S0 state. For 1-H and 1-D, there are 15 major vibrational modes that contribute significantly to the total reorganization energy of the S1 → S0 vibrational relaxation (Fig. 2e, blue and red lines), five of which mainly contain carbazole ligand vibrations (Fig. 2e, red lines; Fig. 2f, modes 1–5). Deuterium substitution on the carbazole ligand (1-H → 1-D) leads to a significant decrease in the vibrational frequency (ωj) of the C-H bond of the carbazole ligand due to the increase in atomic mass (Fig. 2f), while the other major vibrational modes remain almost unchanged (Supplementary Fig. 16, modes 6-7). This is supported by the Raman measurements of 1-H and 1-D. While most of the Raman shifts of both emitters are comparable, shifts at ~745, 1010, 1285 and 1446 cm−1 for 1-H were not observed in the Raman spectrum of 1-D, but shifts in the lower energy regions at ~650, 853, 1201 and ~1329–1340 cm−1 were observed (Supplementary Fig. 17). Since the total reorganization energies (λ) of 1-H and 1-D are similar (λ = 317 meV for both emitters), the smaller the vibrational frequency of 1-D, the higher the effective Huang-Rhys factor (Seff) of 1-D relative to 1-H (S = λ/?ω, where ? is the reduced Planck’s constant; Seff = 0.00783 for 1-D and 0.00758 for 1-H)27,39. According to the energy gap law (Eq. 1)40,41,42, lower vibrational frequencies lead to a reduction of non-radiative decay. This explains why the knr values of 1-D and 2-D are lower than those of 1-H and 2-H in degassed toluene (Supplementary Table 5).
where ΔE is the adiabatic energy difference between the S0 and S1 states. ωM and λM are the vibrational frequency and reorganization energy of the vibrational modes that induce non-radiative decay. l is the number of vibrational modes. C is the effective coupling constant.
TADF-OLED characteristics and operational stability
The electroluminescent (EL) properties of CMA(Cu) emitters were studied by fabricating and characterizing OLEDs based on emitters 1–7. Detailed information on the device structure, auxiliary organic materials, fabrication, and characterization process can be found in the Supplementary Fig. 18, Methods and Supplementary Methods. The EL spectra and EQE-luminance characteristics of OLEDs based on 1–7 are shown in Fig. 3a–d and Supplementary Fig. 19–28. Key device performance data are summarized in Table 1 and Supplementary Table 7.
Fig. 3: OLED characteristics and operational lifetime measurement.
a Normalized EL spectra and b EQE-luminance characteristics of OLEDs based on CMA(Cu) emitter 1–6 in RH host and 7-D in SiCzCz:SiTrzCz2 co-host. c Normalized electroluminescence (EL) spectra and d EQE-luminance characteristics of OLEDs based on emitter 6 in RH host; inset of c displays the electroluminescence image of OLED containing 2 wt% 6. e Operational lifetime measurement of OLEDs with emitters 1–6 in RH host and 7-D in SiCzCz:SiTrzCz2 co-host at L0 of: 11500 cd m−2 for 1-D (4 wt%), 20000 cd m−2 for 2-D (6 wt%), 8500 cd m−2 for 3-H (8 wt%), 20000 cd m−2 for 3-D (2 wt%), 10000 cd m−2 for 4 (4 wt%), 7000 cd m−2 for 5 (2 wt%), 900 cd m−2 for 6 (2 wt%), and 10000 cd m−2 for 7-D (8 wt%).
As shown in Fig. 3a and Table 1, the EL λmax of selected devices containing emitters 1-D, 2-D, 3-H, 3-D, 4, 5, and 7-D are located at 563, 596, 591, 610, 586, 636, and 493 nm, respectively. The maximum EQEs (EQEmax) of devices containing emitters 1-D, 2-D, 3-H, 3-D, 4, and 5 doped in the RH host are 16.4%, 21.2%, 17.5%, 23.1%, 16.5% and 11.4%, respectively (Fig. 3b). The device structures were further optimized by replacing the RH host in the 2-D and 3-D devices with NBP-BC: PCPF-Trz co-hosts and replacing the RH host in devices 4 and 5 with TCTA:TPBi co-hosts, which increased the EQEmax of these four devices to 22.7%, 24.0%, 24.0% and 17.5%, respectively (Table 1). The EQEmax of the device containing the blue emitter 7-D doped in SiCzCz:SiTrzCz2 co-host is 23.5% (Fig. 3b; and Table 1).
For those devices containing red emitters (e.g., 2-D, 3-H, 5 and 6), the EQEmax of the examined devices decreases with increasing emitter doping concentration. For example, when the concentration of emitter 6 increases from 2 wt% to 6 wt% and 10 wt%, EL λmax shifts from 690 (deep red region) to 720 nm and 722 nm (NIR region), while EQEmax decreases from 9.59% to 7.17% and 6.62% (Fig. 3c, d and Table 1). The red-shift in emission energy and decrease in efficiency may be due to the intermolecular interactions between these Cu(I) red emitters in the OLED emissive layer (EML), which can quench excitons at high doping concentrations. Nevertheless, among NIR OLEDs based on earth-abundant Cu(I) complexes, the NIR device containing 6 wt% 6 achieved the highest EQE value and was competitive with Au-based NIR OLEDs23,41,43,44.
Notably, OLEDs based on emitters 1–7 exhibited good operational stability under our laboratory conditions. At initial luminance (L0) of 11500, 20000, 8500, 20000, 10000, 7000, 900, and 10000 cd m-2, the operational lifetimes at 95% of L0 (LT95) are 17.1, 16.6, 32.1, 16.8, 26.2, 5.98, 60.9 and 0.42 hours for the devices with emitters 1-D, 2-D, 3-H, 3-D, 4, 5, 6, and 7-D, respectively (Fig. 3e; and Table 2). Using the exponential decay model with the acceleration factor (n), the LT95 lifetimes at 1000 cd m-2 were estimated to be 1354 hours (1-D), 2398 hours (2-D), 1682 hours (3-H), 3582 hours (3-D), 1407 hours (4), 157 hours (5), and 13.6 hours (7-D) (Table 2; and Supplementary Fig. 29; Supplementary Methods).
Notably, the OLEDs containing 2-D emitters doped in RH hosts exhibited higher EQEmax values and had an operational lifetime more than four times longer than that of 2-H (EQEmax of 14.4% and 21.2% for 2-H and 2-D, respectively; LT95 values of 532 h and 2398 h for 2-H and 2-D, respectively; Tables 1 and 2)22. The same trend was observed for 3-H and 3-D (Tables 1 and 2). These results show that deuterium-substituted carbazole ligands have a significant impact on improving the efficiency and stability of CMA(Cu)-based OLEDs. Additional experiments were also performed to further evaluate the differences in the photophysical properties and stability of 2-H and 2-D. On the one hand, in order to simulate the EML of OLED and evaluate the photophysical properties of these emitters under OLED conditions, films doped with 4 wt% and 8 wt% 2-H and 2-D in the RH host were prepared by vacuum-deposition (VD) method. It was found that 2-D in RH exhibited higher Φem and lower knr than 2-H (Supplementary Table 5), which can be attributed to the use of deuterated carbazole ligands to reduce the vibrational frequency (Fig. 2e, f). This is why 2-D-based OLEDs have higher device efficiency. On the other hand, the Td of the deuterated emitter (1-D: 337 oC; Supplementary Table 3) is slightly higher than that of the non-deuterated emitter (1-H: 325 oC)22. The calculated bond dissociation energy (BDE) of the C-D bond in 1-D is, on average, 8.5 kJ/mol higher than that of the corresponding C-H bond of 1-H (Supplementary Fig. 30), indicating that the C-D bond is stronger than the corresponding C-H bond. In addition, we further performed photo-/electro-chemical stability tests to evaluate the intrinsic stability of these deuterated and non-deuterated emitters (Supplementary Fig. 31–32)27,45,46. As shown in Supplementary Fig. 31a, the absorbance of the 2-H LLCT band decreased by 31% after continuous irradiation with a yellow LED for 36 min. In comparison, 2-D degraded more slowly, with a 28% decrease in absorbance after 36 min (Supplementary Fig. 31b). As shown in Supplementary Fig. 32a–b, after 60 voltametric cycles of repetitive scans, the Epa of 2-H and 2-D decreased by 35.5% and 28.9%, respectively. This indicates that the 1e-oxidized species of 2-D has higher electrochemical stability than that of 2-H. In contrast, the Epc of 2-D and 2-H both decreased by 11% after 60 voltametric cycles (Supplementary Fig. 32c–d). These stability tests show that 2-D is more stable than 2-H, which may explain the significantly extended operational lifetime of 2-D-based OLEDs.
In addition, OLEDs based on emitters 3-H and 4 also exhibited good device stability, with operational lifetimes approximately three times longer than that of OLEDs based on 2-H (Table 2). Compared with 2-H, the 3-H doped in RH VD film mimicking the OLED EML exhibits a shorter τem and a larger kr (Supplementary Table 5), which is beneficial to improve the stability of OLEDs. This can be attributed to the fact that complex 3-H adopts the DMIC ligand with π-extended fluorene substituent, which has a larger electron-hole distance (Fig. 2d). The 3-D emitter with deuterated π-extended carbazole ligands enables OLEDs with a LT95 lifetime of 3582 h at 1000 cd m-2, which is the longest lifetime among the Cu(I) TADF OLEDs studied in this work and comparable to high-performance OLEDs using noble metal emitters (Supplementary Table 8). In contrast, the high device stability of the 4-based OLEDs may be attributed to the steric effect provided by the bulky 1,8-methyl substituted carbazole ligands (Supplementary Discussion and Supplementary Fig. 33)26.
Förster energy-transfer (FRET) and TSF-OLEDs
Although TADF OLEDs employing Cu(I) emitters 1–7 exhibit high efficiency and good operational stability, their EL spectra have large full-width-half-maximum (FWHM) values (> 100 nm for the yellow and red OLEDs; Table 1), showing unsatisfactory color purity. To address this issue, we constructed a ternary system, selecting 1-D, 2-D, or 3-H as the sensitizer, the previously reported α-NAICZ47 as the fluorescent emitter, and RH as the host (Fig. 4a).
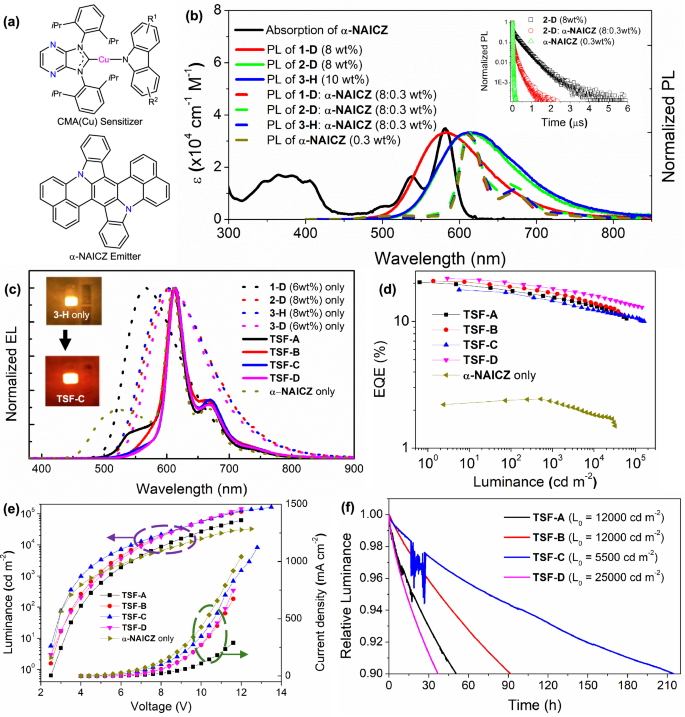
Fig. 4: TSF-OLED characteristics and operational lifetime measurement.
a Chemical structures of CMA(Cu) sensitizers and α-NAICZ emitter. b Absorption spectrum of α-NAICZ in DCM and normalized photoluminescence (PL) spectra of binary system of CMA(Cu) sensitizers in RH and α-NAICZ emitter in RH, and ternary system of CMA(Cu) sensitizers and α-NAICZ emitter in RH; inset displays the PL decay of 2-D/α-NAICZ/RH ternary system, 2-D/RH or α-NAICZ/RH binary systems. c Normalized electroluminescence (EL) spectra, d EQE-luminance characteristics, e luminance-voltage (left) and current density-voltage (right) characteristics of TSF-OLED with CMA(Cu) complex: α-NAICZ and OLEDs with CMA(Cu) complexes or α-NAICZ only; insets of c display the electroluminescence images of OLEDs containing 2 wt% 3-H only (upper) and TSF-C (down). f Operational lifetime measurement of TSF-OLEDs; L0 refer to the initial luminance. Composition of TSF-OLEDs: TSF-A contains 1-D (6 wt%):α-NAICZ (0.2 wt%); TSF-B contains 2-D (8 wt%):α-NAICZ (0.4 wt%); TSF-C contains 3-H (8 wt%):α-NAICZ (0.3 wt%); TSF-D contains 3-D (6 wt%): α-NAICZ (0.3 wt%).
The Förster energy transfer (FRET) process of the Cu/α-NAICZ/RH ternary system was investigated by studying the emission properties of the corresponding VD thin films. The absorption spectrum of α-NAICZ (in DCM)47 overlaps well with PL spectra of doped 1-D, 2-D, and 3-H in RH VD films, resulting in large spectral overlap integrals J(λ) of 6.8–11.6 × 1014 M-1 cm−1 nm4. The Förster radius distance of this ternary system was estimated to be 3.92–4.41 nm, and the theoretical FRET rate constant (kFRET) was 3.1–6.3 × 108 s−1 (see Supplementary Discussion for details). The FRET efficiency (EFRET) reached 0.99, indicating that the ternary system can achieve a highly efficient FRET process. It is noteworthy that all Cu/α-NAICZ/RH ternary films exhibit narrowband emission with λmax at 612–616 nm, which is the same as the emission of α-NAICZ/RH binary film and thus has higher color purity compared with the broadband emission of Cu/RH binary films (Fig. 4b). The emission quantum yields of these sensitized thin films were 0.54–0.79 (Supplementary Table 5). In addition, the delayed fluorescence lifetimes of these Cu/α-NAICZ/RH ternary films are shorter than those of the corresponding Cu/RH binary films (Fig. 4b, inset; Supplementary Table 5). This helps reduce harmful side reactions such as triplet-triplet annihilation and improves the operational stability of the device.
Based on these energy transfer studies, TSF-OLEDs were fabricated by doping 0.2–0.4 wt% α-NAICZ into the EML of Cu-based devices. To optimize the device performance, we introduced different concentrations of Cu complexes into these TSF-OLEDs. Figure 4c–e and Supplementary Fig. 34 show the EL spectra and OLED characteristics of these TSF-OLEDs, as well as fluorescence OLEDs containing only α-NAICZ. The addition of α-NAICZ effectively narrowed the FWHM of the EL spectrum from 103–122 nm to 33–38 nm, showing that the energy transfer from the Cu(I) sensitizer to the α-NAICZ emitter was quite efficient (Table 1 and Supplementary Table 7).
Taking TSF-OLEDs using sensitizer 2-D as examples, the FWHM of the EL spectrum of TSF-E containing 4 wt% 2-D was 38 nm (Supplementary Fig. 34 and Supplementary Table 7). When the concentration of 2-D increased to 6-8 wt% (TSF-B and TSF-F), the FWHM of the EL decreased to 37 nm, showing a more efficient energy transfer from 2-D to α-NAICZ. Thus, TSF-B and TSF-F achieved higher EQEmax values (20.9% and 20.4%, respectively) compared to TSF-E with an EQEmax of 17.1% (Table 1 and Supplementary Table 7). It is noteworthy that the efficiency of TSF-OLEDs increases with the increase of 2-D concentration (EQEmax of 17.1% for TSF-E containing 4 wt% 2-D, and 20.9% for TSF-B containing 8 wt% 2-D). In contrast, the efficiency of the 2-D-based OLED decreases as the 2-D concentration increases from 4 to 8 wt% (Table 1 and Supplementary Table 7). This is because the efficient energy transfer from Cu(I) complex to α-NAICZ reduces the population of excited states of Cu(I) complex, thereby alleviating the exciton quenching of Cu(I) complex at high concentration.
At L0 of 1000 cd m-2, the estimated LT95 values of TSF-A, TSF-B, TSF-C and TSF-D were 2190, 2805, 1508, and 3689 hours, respectively (Fig. 4f, Table 2, and Supplementary Fig. 35). These values are comparable to the corresponding LT95 values of 1354, 2398, 1682, and 3582 hours for OLEDs using only 1-D, 2-D, 3-H, and 3-D. To the best of our knowledge, Cu(I) emitter-sensitized OLEDs have not previously achieved simultaneously high efficiency and such a long device lifetime. This work highlights the great promise of earth-abundant metal-based OLEDs as efficient and stable alternatives to precious Ir(III)-/Pt(II)-based OLEDs.
Methods
Photophysical measurements
Ultraviolet-visible light (UV-vis) absorption spectra were recorded on a Hewlett-Packard 8453 diode array spectrophotometer or Agilent Cary 60 UV-Vis Spectrophotometer. Steady-state emission and excitation spectra of samples in solution, or glassy state were recorded on a Horiba SPEX Fluorolog 3 spectrofluorometer. Absolute emission quantum yields of solutions and thin-film samples were recorded on a Hamamatsu Quantaurus-QY Absolute PL quantum yields measurement system C11347-11. Emission lifetimes (τ) were measured with a Quanta Ray GCR 150-10 pulsed Nd:YAG laser system (pulse λexc = 355 nm), Beamtech Nimma-600, or Quantaurus-Tau Fluorescence lifetime spectrometer (C16361-01). For measurements in solution at room temperature, samples were placed in two-compartment cells consisting of a 10 mL Pyrex bulb and a quartz cuvette with 1 cm path length. The cells were sealed from the atmosphere with Rotaflo stopcocks. Solutions were degassed in a high-vacuum line by five freeze-thaw-pump cycles. Thin-film samples were prepared by drop-casting solutions of a complex in toluene with 1,3-bis(N-carbazolyl)benzene (mCP) onto clean quartz plates, and solvents were evaporated at 60 °C. Alternatively, thin-film samples were prepared by thermally depositing the emitter and RH host material at a rate of 0.5 Å s−1 using co-deposition technology in a Kurt J. Lesker SPECTROS vacuum deposition system with a base pressure of 10-7 mbar.
Theoretical calculations
DFT/TD-DFT calculations including geometry optimization, frontier molecular orbital diagram calculation, ΔEST value calculation, and ground state and excited state frequency calculation were performed using the Gaussian 16 program package48. The hybrid functional PBE049,50 with D3 version of dispersion correction with Becke-Johnson damping (D3BJ)51,52 were used for calculations. The 6-31 G* basis set was applied for all atoms except gold, which was described by a Stuttgard/Dresden SDD basis set and the associated pseudopotentials53. Solvent effects were considered by means of the polarizable continuum model (PCM) with toluene as solvent54. The triplet excited state structures were optimized by the restricted TD-DFT method. The topographical steric maps and buried volumes with spherical shape were obtained using the SambVca 2 web tool55. The buried volume and the frontier molecular orbitals were calculated using the optimized coplanar structures in the ground state. The hole and electron distribution, the overlap between hole and electron wavefunctions (Oh,e), and the distance between centroids of hole and electron (Δr) were calculated using the program Multiwfn 3.8(dev)37. The reorganization energies (λ) of effective modes and the corresponding Huang-Rhys factor (S) were determined using the DUSHIN program developed by Reimers56. The hole and electron distributions and the vectors of vibrational modes were rendered by the VMD 1.9.3 software57.
Fabrication and characterization of OLEDs
Indium-tin-oxide (ITO) coated glass with a sheet resistance of 10 Ω/sq was used as the anode substrate. Before film deposition, patterned ITO substrates were cleaned with detergent, rinsed in de-ionized water, acetone, and isopropanol, and then dried in an oven for 1 h in a cleanroom. The slides were then treated in an ultraviolet-ozone chamber for 5 min. The OLEDs were fabricated in a Kurt J. Lesker SPECTROS vacuum deposition system with a base pressure of 10-7 mbar. In the vacuum chamber, organic materials were thermally deposited in sequence at a rate of 0.5 Å s−1. The doping process in the EMLs was realized using co-deposition technology. Afterward, Yb (1 nm), or Liq (2 nm) or LiF (1.2 nm) and Ag (100 nm) or Al (100 nm) were thermally deposited at rates of 0.02 and 0.2 nm s−1, respectively. The film thicknesses were determined in situ with calibrated oscillating quartz-crystal sensors.
Current density-brightness-voltage characteristics, EL spectra, and EQE of EL device were obtained by using a Keithley 2400 source-meter and an absolute external quantum efficiency measurement system (C9920-12, Hamamatsu Photonics). All devices were encapsulated in a 200-nm-thick Al2O3 thin film deposited by atomic layer deposition (ALD) in a Kurt J. Lesker SPECTROS ALD system before measurements.
Data availability
The data that support the findings of this study are provided in the Supplementary Information, Supplementary Data 1 file, and Source Data file. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition numbers CCDC 2419364 (2-D), CCDC 2252752 (3-H), CCDC 2419367 (3-D), CCDC 2346434 (4’), and CCDC 2237011 (6), and can be obtained free of charge from CCDC via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.