SignificanceMagnetic field quantum sensors, including superconducting quantum interferometers, laser-pumped atomic sensors (LPAS), and nitrogen-vacancy centers in diamonds, utilize quantum systems or effects to precisely measure magnetic fields. Laser-pumped atomic magnetometers, known for their high sensitivity, compact size, low power consumption, and ease of maintenance, represent a rapidly evolving research area. LPAS are applied in nuclear magnetic resonance (NMR) for obtaining more accurate magnetic resonance spectra of materials and for measuring samples under unique conditions. This expands the detection and analytical capabilities in discerning the fine structure of biological and chemical substances. They are anticipated to serve as an effective complement to high-field NMR techniques.ProgressNMR based on LPAS has been developed rapidly in recent years. Researchers have integrated hyperpolarization technology, sample transmission, and coding technology with high sensitivity and broad bandwidth LPAS. This integration enables the performance of zero- to ultralow-field NMR on various chemical samples. It allows for the acquisition of the samples’ zero- to ultralow-field NMR spectra and facilitates the theoretical analysis of these spectra. Additionally, the researchers have successfully conducted zero- to ultralow-field NMR measurements of chemical reactions within metal sample tubes. This advancement permits non-destructive, real-time monitoring of the polarizability of hyperpolarized samples. Furthermore, combining this with image coding in NMR, zero- to ultralow-field magnetic resonance imaging (MRI) of the human brain and hand has been realized.Conclusions and ProspectsLPAS method and technique are crucial for realizing zero- to ultralow-field NMR and MRI. LPAS offers low manufacturing costs, simple maintenance, easy miniaturization, and boasts an ultra-narrow linewidth with high sensitivity of approximately fT/Hz1/2. Utilizing LPAS technology has transformed zero- to ultralow-field NMR into a powerful tool, especially in fields such as biochemistry. Building on this, the integration of nuclear spin polarization enhancement technologies and sample transport technologies addresses the challenges of performing NMR and MRI in the thermal polarization measurement environment of the sample at zero- to ultralow fields. This integration effectively broadens the application scope of LPAS-based NMR and MRI methods and technologies. By combining these with zero- to ultralow-field NMR coding techniques, high spectral and imaging resolutions are achievable. Additionally, there are fewer restrictions on the materials of the substances being detected, offering innovative directions for the development of NMR measurement and MRI methods in biomedicine and chemical materials.The development of nuclear magnetic resonance spectrometers based on LPAS has progressed rapidly. However, there are still areas for improvement, such as enhancing the analysis of zero- to ultralow-field NMR spectra, improving the measurement resolution of zero- to ultralow-field NMR spectrometers, and achieving further miniaturization of these spectrometers. Zero- to ultralow-field NMR spectroscopy necessitates the integration of the physical and chemical information of the sample being tested and detailed analysis using controlled coded pulses. The resolution of the spectrometer can be enhanced through the application of hyperpolarization technology and by increasing the sensitivity of LPAS. Miniaturization is a key development trend for zero- to ultralow-field NMR spectrometers. The current size of LPAS has been reduced to centimeter scale, and with advancements in new materials and manufacturing technologies, there is potential for even further miniaturization.
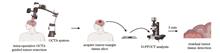
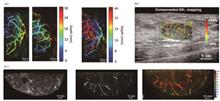
Progress We introduced a series of research efforts to advance the intraoperative application of optical coherence imaging. Vascular characteristics are an important basis for intraoperative pathological assessment. We first introduced OCT angiography with adaptive multi-time intervals, which proposes a time-efficient scanning protocol by adaptive optimization of the weights of different time-interval B-scan angiograms. This novel OCTA technique achieved better performance, with a visible vascular density increase of approximately 67% and a signal-to-noise ratio enhancement of approximately 11.6% (Figs. 2 and 3). In the context of intraoperative applications, we introduced robot-assisted OCTA, which integrated a high-resolution OCT system with a 6-degree of freedom robotic arm (Fig. 4). Robot-assisted OCTA can achieve wide-field imaging of artificially determined scanning paths. High-resolution vascular imaging of the mouse brain by robot-assisted OCTA successfully confirmed the effect of unevenly distributed resolution and fall-off caused by the large-curvature sample (Fig. 5). Thereafter, we introduced a microscope-integrated OCT system that can be well integrated with current intraoperative equipment and does not need to pause the surgical process (Figs. 6 and 7). Providing real-time tissue depth information to a doctor can help improve their decision-making ability in delicate surgical procedures such as ophthalmology and nervous system surgery. Intraoperative three-dimensional (3D) real-time imaging requires an OCT system with high imaging and processing speeds. Thereafter, we introduced the 10.3 MHz ultra-high speed scanning laser with stretch pulse mode-locked based on polarization isolation (Fig. 8), which employs a simple and low-cost approach to suppress the transmitted light and achieves an effective duty cycle of ~100% with only one CFBG and no need for intra-cavity semiconductor optical amplifier (SOA) modulation, extra-cavity optical buffering, and post amplification (Fig. 9). Real-time 3D OCT imaging is necessary for practical intraoperative applications, and a series of studies have been conducted to achieve this goal. A home-built 3.28 MHz FDML based OCT system combined with GPUs (NVIDIA, GeForce GTX690, and GeForce GTX680, USA) achieved real-time processing and visualization of 3D OCT data (Fig. 10). The imaging range and longitudinal resolution can be flexibly adjusted by changing the spectral range of the output.Although OCT offers high-quality structural and vascular imaging, it lacks cellular resolution, which limits detailed tumor analysis. Dynamic full-field OCT (D-FFOCT) is an optically active rapid pathological imaging technology based on array interference detection that captures subcellular metabolic motion at millisecond temporal and nanometer spatial scales, and significantly enhances tumor diagnostics by providing detailed insights beyond conventional OCT capabilities (Fig. 11). Normal and diseased tissues can be accurately distinguished by analyzing the temporal characteristics of dynamic signals, such as amplitude, frequency, and standard difference. Through the use of high-power objective lenses and broadband light sources, the resolution can reach sub-microns, and as an imaging tool for intraoperative tissue sections, it is fast, easy (no freezing or staining is required), and highly accurate. Freshly isolated mouse brain glioma sections were imaged using the D-FFOCT system, which showed a clear boundary, distinct cell structure, and dynamic intensity between the glioma and normal brain tissue (Fig. 12).Conclusions and Prospects Advancements in OCT technology, including the significantly increased sweep speed of the light source, improvement of the probe for the intraoperative scene, optimization of the blood flow algorithm, and high-speed data processing capability supported by the GPU, make real-time intraoperative 3D tomography possible. D-FFOCT imaging with a cell-resolving ability is an important step forward in the timely pathology of tumor resection. The integration of advanced OCT technologies into clinical practice heralds a new era of precision medicine in which surgical accuracy is significantly enhanced and tumor recurrence is minimized. Future studies should focus on further refining OCT capabilities, integrating these advanced technologies to improve clinical practicability, expanding their applications across different types of cancer, and integrating AI to automate and enhance diagnostic accuracy. This vision foresees OCT not only as a tool for improved surgical interventions but also as a pivotal element in the broader strategy of personalized and targeted treatment approaches, offering a beacon of hope for more effective cancer management and patient recovery paths. The ultimate goal is to establish OCT as an indispensable tool for tumor surgery and management, revolutionizing patient care and outcomes.SignificanceOptical coherence tomography (OCT) plays a pivotal role in medical imaging, particularly in enhancing the tumor resection accuracy. The significance of this technology lies in its ability to improve patient prognosis by providing real-time, detailed visualization of tumor boundaries and invasiveness, thereby reducing recurrence rates and aiding the precise removal of malignant tissues.
SignificanceSince 2020, breast cancer has emerged as the most prevalent cancer globally and a leading cause of cancer-related deaths among women. Affected by various genetic or environmental carcinogenic factors, breast cells undergo irreversible gene mutations, initiating the uncontrolled proliferation of malignant cells that crowd into clusters to form breast tumors. The in-situ tumors induce local tissue hypoxia in their internal and surrounding areas, leading to vascular hyperplasia, which propels the growth of cancer cells and their invasion into normal tissues.Medical imaging is the primary tool for breast cancer screening, diagnosis, and treatment assessment. Early screening plays important roles in reducing mortality; accurate diagnosis is essential for effective treatment; and treatment assessment is critical to provide timely feedback and prognosis of cancer responses. Conventional imaging methods for breast cancer, such as mammography, ultrasonography, and magnetic resonance imaging, though widely used in clinics, exhibit limitations including low diagnostic specificity, slow imaging speed, ionizing radiation, or the need of contrast agent injection. For instance, more than 75% of patients receive benign biopsy results after ultrasound diagnosis. Furthermore, current imaging modalities lack the capacity to provide real-time monitoring, evaluation, and prognosis of the cancer responses during neoadjuvant therapy. New imaging modalities with complementary advantages are crucial to address the evolving clinical demands.Photoacoustic imaging (PAI) is an emerging technology in the biomedical imaging field and has garnered significant attention owing to its exceptional performance. In addition to its high imaging speed, high spatiotemporal resolution, ionizing-free radiation, and abundant penetration, PAI can provide rich functional optical contrast to reveal physiological characteristics of the tumor microenvironment underneath the skin.ProgressMultiple research groups in the PAI field have achieved notable technical breakthroughs for breast cancer screening, diagnosis, and treatment assessment. Regarding early screening, advanced PAI devices have been developed based on customized ultrasonic arrays. These devices aim to detect physiological characteristics such as vascular proliferation, increased hemoglobin concentration, and abnormal blood oxygen saturation in breast tumor areas through entire breast scanning. Some teams have explored the integration of PAI and ultrasonography, utilizing the complementary anatomical information. As concerns breast tumor diagnosis, numerous clinical studies have demonstrated that physiological characteristics in the microenvironment of a tumor can improve the distinction between benign and malignant breast tumors, facilitating accurate BI-RADS classification and reducing the chance of benign biopsy. The high imaging contrast of PAI also enables the guidance of breast sentinel lymph node biopsy with better clearance. While the combination of PAI with exogenous contrast agents and molecular probes is still in the preclinical stage, it holds the potential for more specific diagnosis in future. Regarding treatment assessment, PAI proves efficient and safe in recording physiological dynamics of the cancer microenvironment in response to therapy, offering crucial prognostic information and seamless feedback to the treatment. In addition, the label-free nature of ultraviolet PAI also provides H&E-like images without the need for staining, exhibiting early promise for accurate and rapid detection of tumor margins intraoperatively.Conclusions and ProspectsRegardless of the numerous advantages and multiple niche applications, PAI still faces several challenges to achieve wide clinical usage. First, the spread of PAI technologies depends on the established standards of system design, operation, and data processing to reduce the significant performance disparities among devices developed by different teams. Second, several feasibility studies have been conducted in the PAI field but large-scale clinical studies are still lacking. The PAI indicators revealed from breast cancer images have not been systematically documented or incorporated into clinical practice. Third, a gap still exists between the technical teams and clinical needs. For instance, while three-dimensional PAI exhibits better image clarity for lesion measurement, clinical practices and diagnostic analyses still heavily rely on real-time two-dimensional sectional imaging. Accordingly, to further establish its clinical value, PAI researchers need to evolve from scattered and small-scale feasibility studies to large-scale clinical trials addressing fundamental medical questions. This involves improving existing diagnostic and treatment methods and ultimately integrating them into the existing clinical framework.
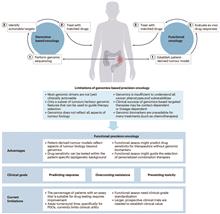
The complexity of tumor biology is a multifaceted challenge that is governed by the intricate relationship among genetic mutations, epigenetic alterations, and the tumor microenvironment. Tumors are not static—they evolve through a series of genetic and epigenetic changes that enables them to evade the host’s immune system and resist the effects of various treatments. The tumor microenvironment, which comprises a diverse array of cell types, extracellular matrix components, and signaling molecules, significantly affect tumor growth, metastasis, and response to therapy. This renders it difficult to develop comprehensive treatment plans that can effectively target the specific characteristics of each tumor.Optical microscopy imaging technologies have been adopted widely in precision oncology as they can address the challenges posed by the complexity of tumor biology. These technologies allow one to visualize and analyze tumor tissues and cells with high resolution, thus enabling quantitative and spatially localized analysis of genomic, proteomic, and metabolomic information. This level of detail is critical for identifying patient-specific molecular characteristics and biochemical abnormalities for developing targeted treatment strategies.The significance of optical microscopy imaging in precision oncology is manifold. First, it bridges the difference between the genomic and phenotypic aspects of cancer, thus allowing for a more nuanced understanding of tumor behavior and response to therapy. Second, it enables the identification of biomarkers that can predict treatment response, thus providing guidance in selecting the most appropriate treatments for individual patients. Third, the non-invasive nature of these imaging techniques allows for the repeated monitoring of tumor progression and response to treatment, thereby facilitating real-time adjustments to treatment strategies as necessary.The potential of optical microscopy imaging to transform cancer treatment is substantial. By providing detailed, patient-specific information, these imaging techniques can facilitate the development of more effective and less-toxic treatment regimens. This personalized approach can improve patient outcomes by increasing the efficacy of therapies and reducing the incidence of adverse effects. Furthermore, the ability to monitor treatment response in real time can facilitate more informed clinical decision-making, thus potentially improving the overall survival rates and quality of life of patients with cancer.In conclusion, the integration of optical microscopy imaging into precision oncology is a significant advancement in cancer treatment. Optical microscopy imaging technologies are effective for understanding the complex biology of tumors and for guiding the development of personalized treatment strategies. As research in this field continues to progress, the potential for optical microscopy imaging to revolutionize cancer diagnosis and treatment will be immense, thus affording more targeted therapies and better patient outcomes in the future. The continued evolution of these technologies is crucial for bridging the disparity between genomic research and clinical practice, thus ultimately resulting in more effective and personalized cancer treatments.Progress Optical microscopy imaging techniques have progressed significantly in the field of precision oncology and can provide a comprehensive view of tumor characteristics. Auto-fluorescence (AF) imaging has been utilized to monitor metabolic activities within tumors and offers label-free insights into drug responses and cellular metabolism (Fig.5). Second harmonic generation (SHG) imaging has been pivotal for analyzing the extracellular matrix (ECM), particularly collagen fiber organization, which is crucial for understanding tumor invasion and metastasis (Fig.7). Coherent Raman scattering (CRS), in particular stimulated Raman scattering (SRS), has emerged as an effective tool for imaging tumor metabolites without requiring labels. SRS has been instrumental in revealing metabolic heterogeneity, which is vital for identifying therapeutic targets and understanding cancer-cell metabolism (Fig.8). Mid-infrared photothermal (MIP) imaging has demonstrated its potential in assessing drug pharmacokinetics and pharmacodynamics by imaging the distribution of drugs within cells and tissues at a deep cellular level (Fig.9). Furthermore, multiplex immunofluorescence (mIF) and fluorescence insitu hybridization (FISH) have been employed for immunophenotyping (Fig.4) and genetic analysis (Fig.6), respectively, to characterize the immune microenvironment and detect gene amplifications. These techniques, as summarized in Table 1, collectively contribute to the increasing number of tools available for the characterization of tumors and the optimization of targeted therapies, thus ultimately improving patient outcomes in cancer treatment.Conclusions and Prospects Optical microscopy imaging is becoming essential in precision oncology as it allows one to understand the relationship between tumor genetics and phenotypes. As the field progresses, the integration of these imaging techniques into clinical settings will become more evident, which will significantly improve cancer diagnostics and treatment. Future studies shall be conducted to render this technology more accessible by reducing equipment costs and enhancing imaging methodologies, thereby solidifying its key role in precision oncology.SignificancePrecision oncology is imperative for accommodating the distinct journey of each cancer patient, which is determined by the unique genetic, molecular, and cellular profiles of individual tumors. This shift from a general treatment model to a personalized approach is driven by the recognition that each patient with cancer presents a distinct set of challenges that must be addressed to achieve optimal therapeutic outcomes and prognostic accuracy. The conventional methods of cancer treatment, which typically involve generalized therapies, are deficient owing to the heterogeneity of tumors and the dynamic nature of cancer progression.
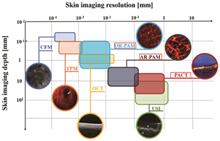
SignificanceSkin diseases are common human conditions, and their detection and diagnosis are necessary. Because of the influence of doctors' subjectivity and skin trauma, traditional detection methods are inadequate for accurate and effective diagnosis of skin diseases. Therefore, skin imaging techniques are gradually being used for diagnosis. The photoacoustic imaging technique is an emerging imaging method that combines the high contrast of optical imaging with the deep imaging advantages of ultrasound imaging. Photoacoustic images provide structural and functional information to assist doctors in diagnosing diseases and to improve the accuracy of assessment and treatment. Photoacoustic skin imaging technology can satisfy imaging requirements of different hardware configurations, and its variety of hardware forms ensures that the technology can achieve microscopic and macroscopic imaging, with the potential to respond to diverse clinical needs. By using this technology, melanin and hemoglobin can be detected when capturing images of melanin particles and microvessels at different depths in the palm of the human hand, which can be used for diagnosing pigmented and vascular skin diseases. Photoacoustic skin imaging provides high-resolution images of all skin layers, which is crucial for the early diagnosis and evaluation of skin diseases. Therefore, this paper reviews the systematic classification of existing photoacoustic skin imaging modalities and the performance enhancement methods of image reconstruction algorithms, describes several applications of photoacoustic imaging in clinical human research, and analyzes the advantages and clinical potential of photoacoustic skin imaging as an emerging imaging technology. Thus, readers can gain a detailed and comprehensive understanding of photoacoustic skin imaging technology.ProgressThis paper reviews and summarizes the photoacoustic skin imaging technology. First, photoacoustic skin systems are classified based on the imaging modality. Existing photoacoustic skin imaging systems are divided into photoacoustic microscopic and other photoacoustic skin imaging systems. The latter includes photoacoustic tomography and ultrasound/photoacoustic multimodal-imaging systems. This article summarizes research with superior performance in terms of imaging principles, resolution, imaging depth, scanning modes, and other hardware specifications. The corresponding system components are outlined. Subsequently, the research progress in photoacoustic microscopic skin imaging systems and other photoacoustic skin imaging systems is summarized in terms of comparison of the overall system performance.In studies on photoacoustic microscopy imaging systems, significant progress has been achieved in improving photoacoustic dermoscopy systems, in-vivo skin microimaging, and multiscale skin microimaging, resulting in advancements in system performance (Table 1). Moreover, for other types of photoacoustic skin imaging systems, studies have focused on various aspects, such as photoacoustic tomography of subcutaneous blood vessels in the extremities, three-dimensional photoacoustic tomography, diode laser-based skin tomography systems, and ultrasound/photoacoustic multimodal imaging systems. These investigations lead to noteworthy research outcomes and enhancements in the hardware performance of skin imaging systems (Table 2). In addition, the inclusion of commercial skin imaging systems in the listings validates the practical application value of photoacoustic skin imaging systems.This paper summarizes existing methods and strategies for enhancing the performance of photoacoustic imaging systems, with a focus on advancements in reconstruction algorithms. The analysis categorizes and discusses these methods based on three main aspects: imaging resolution enhancement algorithms, imaging depth enhancement algorithms, and noise removal algorithms. Each category is analyzed chronologically, starting with an overview of conventional and pivotal performance enhancement algorithms. Subsequently, the discussion encompasses performance enhancement algorithms that integrate deep-learning techniques. Finally, existing specialized algorithms are discussed.This paper summarizes research on the clinical application of photoacoustic skin imaging technology, classifies skin diseases into two categories (skin cancer and other skin diseases), and summarizes the photoacoustic skin imaging methods for detecting typical diseases. On the one hand, the research teams are currently focusing on detecting melanin and collagen content in the detection and investigation of skin cancer. On the other hand, the imaging of blood vessel shape and distribution pattern can be used as a criterion to determine whether the detected area is diseased and to identify the lesion boundary. In this paper, other skin diseases are classified as inflammatory, vascular, or pigmented according to their causative factors. The pathological features of the diseases and detection methods based on photoacoustic skin imaging technology are described using typical diseases as examples. A summary of the clinical applications of this technology for diverse skin diseases demonstrates its unique advantages and potential for clinical applications.The concluding section of this article highlights the prevailing challenges of photoacoustic skin imaging and outlines the corresponding research directions aimed at addressing these issues. These challenges include resolving the issue of dynamic changes in clinical data, advancing multimodal imaging capabilities, developing user-friendly imaging devices, and establishing standardized imaging protocols and data analysis techniques.Conclusions and ProspectsPhotoacoustic skin imaging is an emerging technique with several advantages, such as excellent imaging quality, cost-effectiveness, tissue safety, and promising clinical potential. Photoacoustic imaging is anticipated to become widely used in the future for clinical skin examinations. Further exploration and development of photoacoustic skin imaging technology are required to advance its clinical applications and facilitate its integration into medical practice.
SignificanceViruses are the primary cause of many infectious diseases, including influenza, high-mortality lower respiratory tract infections, diarrhea, tuberculosis, HIV infection, dengue fever, hepatitis B, and more. These diseases can cause severe damage to various systems in the human body and can even lead to life-threatening conditions. The outbreak of infectious viruses poses a significant challenge to public healthcare systems. Early and accurate virus diagnosis is crucial in preventing virus spread, especially in the absence of specific vaccines or effective medications. Existing traditional detection methods often require complex equipment and the expertise of skilled operator. Hence, it becomes challenging to conduct large-scale testing in rapidly spreading virus-infected areas.Surface-enhanced Raman scattering (SERS) technology is an ultra-sensitive vibrational spectroscopy technique, which is used to detect plasmonic nanostructures on the surface or near-surface molecules. Due to its fast response, strong specificity, and non-invasive detection characteristics, SERS has been widely used in surface and interface studies, chemical and biosensors, biomedical monitoring, trace analysis, electrochemical reactions, and catalytic reactions. Specifically, in virus detection, it exhibits extremely high detection sensitivity, enabling rapid and accurate detection of minute virus particles. Based on the analysis of virus spectral features, SERS technology can differentiate between different types of viruses, including subtypes and variants. This high specificity leads to a unique advantage in virus tracing, classification, and epidemiological research, which is crucial for the rapid screening of early virus infections and facilitating timely medical intervention. This review systematically summarizes the research progress and potential applications of SERS technology in virus detection over the past two years, considering factors such as the genetic material of the virus, virus types, and the extent of impact (Fig.1).ProgressInitially, this study categorizes viruses based on their genetic material, focusing on recent efforts to detect RNA and DNA viruses that threaten human life and health. It offers a comprehensive analysis of both labeled and label-free SERS techniques for detecting these virus types. For RNA viruses, such as SARS-CoV-2, the influenza virus, HIV, and DNA viruses, such as HBV and HPV, label-free detection methods require SERS technology to realize enhanced performance in signal amplification of the detection substrate for direct detection of natural biomolecules without amplification. Notable examples include the trap structure introduced by Yang et al., the nano-flexible substrate by Paria et al., and the semiconductor application by Peng et al., which broaden the application scope of SERS technology. In the realm of SERS signal processing, particularly when combined with machine learning techniques, there is a significant advantage in extracting and analyzing spectral features for identifying potential biomarkers or molecular details in complex and varied samples. The creation of sensitive SERS biological probes in labeling methods is especially critical. Accurately tagging target molecules with Raman signal molecules greatly increases the specificity of the detection platform. For example, Guan et al. employed substrate capture and specific recognition probes for detecting the SARS-CoV-2 antigen, while Su et al. developed SERS labels integrated with CRISPR/Cas technology for the non-amplified detection of target genes. Moreover, the miniaturization and portability of Raman instruments, propelled by technological advancements, are steering SERS toward field applications and real-time analysis, aligning perfectly with the point-of-care testing (POCT) concept. This foundation supports the study’s summary of various initiatives that combine SERS technology with portable Raman instruments. It concludes by offering a summary and outlook on optimization strategies and the current challenges facing the application of SERS technology in virus detection and various POCT settings.Conclusions and ProspectsThe efficacy of SERS technology in virus detection hinges on several critical factors, such as the design of the enhancement substrate, excitation conditions, the properties of labels and analytes, detection devices, and data analysis techniques. The primary aim is to enhance detection speed and sensitivity while simplifying the detection process for more efficient virus identification. To navigate the intricate challenges posed by viral outbreaks, the development of integrated micro-detection chips capable of identifying multiple viruses, paired with compact Raman detection devices, stands as the ideal approach for future POCT of viruses. Furthermore, investigating the integration of SERS technology with other detection methods—such as chemical separation, biological capture, colorimetry, and advanced computational approaches like machine learning, deep learning, and artificial intelligence—can maximize the benefits of diverse technologies. This integration promises the creation of innovative Raman analysis devices that consolidate sample processing, detection, analytical processing, statistical analysis, result dissemination, and display functionalities, catering to the on-site and real-time testing demands across various sectors. We expect that merging SERS technology with compact Raman instruments will usher in a convenient, efficient, and precise optical POCT method for virus screening, classification, infection tracking, and prognosis forecasting.
SignificancePhotodynamic therapy (PDT) is a novel treatment for superficial skin diseases and tumors. The basic treatment involves administering a photosensitizing agent through intravenous injection or other methods, and then stimulating the lesion with a specific wavelength of light. This photodynamic reaction, facilitated by the photosensitizing agent, effectively cures the lesion. PDT uses the photodynamic effect for diagnosing and treating diseases. Its mechanism is based on a photosensitization reaction accompanied by biological effects that includes the participation of oxygen molecules. This process involves irradiating a laser of a specific wavelength to excite a photosensitizer that has been absorbed by tissues, causing it to enter an excited state. Then the photosensitizer in the excited state transfers energy to the surrounding oxygen, resulting in the generation of highly active singlet oxygen. This singlet oxygen undergoes an oxidative reaction with adjacent biological macromolecules, inducing cytotoxicity, and ultimately, causing cell damage and death. Over the past 20 years, PDT has emerged and developed as a new treatment technology for diseases such as esophageal cancer, lung cancer, condyloma acuminatum, acne, and nevus.Compared to traditional tumor therapies such as surgery, chemotherapy, and radiotherapy, PDT offers unique and irreplaceable advantages. It is non-resistant, allowing for repeated treatment. PDT exhibits high therapeutic selectivity towards the lesion, causing little to no damage to healthy tissues, and has only a few toxic side effects. Consequently, PDT is especially suitable for elderly and frail patients who are unable to undergo surgical resection or chemotherapy. In particular, for patients with advanced tumors who have not responded effectively to or are at risk with traditional treatments, PDT is an extremely ideal treatment option.Different types of molecules can be used as photosensitizers; however, many of them face challenges in clinical application, including limited penetration depth, low solubility, dark toxicity, and a high dependence on oxygen concentration. Therefore, more efficient and safer photosensitizers need to be further studied and developed. Currently, the focus of research and development of novel photosensitizers lies in target modification and smart nanomedicine delivery systems to achieve minimally invasive and specific therapy. An excellent photosensitizer should be capable of achieving precise lesion killing at low doses while having a minimal effect on other parts of the body. In the context of PDT, the application of novel photosensitizers is undoubtedly a key factor in further improving the therapeutic effect.ProgressThe earliest photosensitizers used in PDT were hematoporphyrin derivatives, with the main component being dihematoporphyrin ether (DHE). Sodium porphyrinum, marketed by Canadian company QLT (Quadra Logic Technologies Phototherapeutics Inc.), has received approval for the treatment of bladder, esophageal, and lung cancers. It has become the most frequently used photosensitizer in the PDT of non-cutaneous solid tumors. However, it still has certain disadvantages, such as long-lasting skin photosensitivity and low selectivity for lesion tissues. Subsequently, a wider variety of photosensitizers have been developed for treating various diseases (Table 1). There have been many studies on both traditional and novel photosensitizers. Porphyrins, chlorins, phthalocyanines, and bacteriochlorin derivatives have been employed as photosensitizers in clinical use (Table 2). Viscous cycloquinone and metal-ligand anthapurpurin derivatives have entered the clinical research stage as photosensitizers (Table 3). Meanwhile, research focusing on the development of new photosensitizers is also in full swing. Researchers are developing photosensitizers on the nano platform and achieving better drug delivery effects through surface modifications of the photosensitizer. They are also aiming to achieve more accurate PDT through the design of activatable and responsive photosensitizers. Furthermore, they are attempting to overcome the oxygen-depleted microenvironments at tumor sites by developing novel type I photosensitizers and creating photosensitizers more suitable for the treatment of deep solid tumors. Additionally, the combination of PDT with other drugs or therapies, to achieve a better therapeutic effect and reduce drug toxicity and side effects, has also garnered researchers’ interest. Sonodynamic therapy, a derivative of PDT, exhibits higher therapeutic efficiency for deep lesions due to its superior tissue penetration ability. Research on acoustic sensitizer and sonodynamic therapy is also underway.Conclusions and ProspectsPDT is playing an increasingly important role in the treatment of many superficial lesions and cancers. The development of photosensitizers with better treatment effects and fewer toxic side effects has been receiving extensive attention. Researchers have made significant efforts in developing more delicately designed photosensitizers. Many photosensitizers with excellent properties, such as high reactive oxygen quantum yield, high molar extinction coefficient, high maximum absorption wavelength, high targeting ability, low in vivo toxicity, and rapid in vivo clearance, have been advanced to clinical research. Simultaneously, more photosensitizers have received marketing approval, benefiting patients. The development of photosensitizers has advanced the diagnosis and precise regulation of diseases, contributing to the development of precision medicine. With the continuous development of novel photosensitizers, PDT will play a greater role in multiple indications and bring benefits to a larger number of patients.
SignificanceDigital pathology uses digitized pathological images and their features in conjunction with artificial intelligence technology to achieve quantitative characterization of cancerous tissues and assist pathologists in clinical diagnoses. The use of polarized light illumination and polarized light detection can achieve full polarization imaging. Accordingly, the polarization characteristics of each pixel of the image contain abundant microstructural information, especially subcellular super-resolution information, that is difficult to obtain with nonpolarization imaging. Polarization imaging can provide a more effective means for the identification and quantitative characterization of cancerous tissues. This paper introduces Mueller matrix microscopic imaging techniques and comprehensively reviews the latest methods for polarization feature extraction, including supervised learning-based polarization pixel and image feature extraction, unsupervised learning-based polarization pixel clustering, and the extension of annotations through polarization feature templates based on super-pixels, highlighting their potential clinical applications.ProgressMueller matrix imaging provides abundant subcellular-level information on tissue microstructures. The quantitative extraction of polarization features from Mueller pixels is crucial for the clinical application of polarization imaging. In contrast to stain image-based digital pathology, polarization feature extraction through supervised learning offers more abundant microstructural information. However, the reliance on extensive, well-annotated data poses time and labor challenges. Moreover, supervised learning is dependent on pathologists’ prior knowledge, limiting the comprehensive utilization of information from the polarization space. Unsupervised clustering methods facilitate the decomposition of pathological tissues into distinct microstructural subtypes, enhancing the exploration of the rich information embedded in Mueller pixels. Additionally, this approach provides evidence for the ongoing discovery of new physical properties, structural characteristics, and dynamic processes at all levels above the subcellular scale in organisms, including living entities.Conclusions and ProspectsFollowing advancements in molecular biology techniques, the specific identification of molecular components in biological entities is becoming a pivotal tool in biomedical research, thus leading to diverse omics approaches. Polarization-based digital pathology can leverage feature extraction methods developed in various omics approaches. The unsupervised clustering of Mueller pixels quantitatively extracts information at various levels above the subwavelength scale, enabling the integration of label-free, noninvasive, abundant information features of Mueller matrix imaging into novel spatiotemporal omics methods.
SignificanceBreast cancer is the most common cancer diagnosed among women worldwide, which accounts for 11.7% of all new cancer diagnoses in 2020. Breast cancer mortality rates decrease significantly when breast tumor is detected early using imaging tools. As an emerging imaging technique, near-infrared spectral tomography (NIRST) has demonstrated potential in breast imaging owing to its nonionizing radiation and high sensitivity and cost-effectiveness. The aim of NIRST is to resolve three-dimensional images of tissue optical properties and chromophore concentrations from acquired multi-wavelength measurements. Therefore, functional information related to biological tissue can be obtained, which is indistinguishable using current clinical breast-imaging modalities. However, NIRST exhibits poor spatial resolution because of light scattering in biological tissues. NIRST system is the key ingredient for producing NIRST images of high spatial resolution.In recent decades, various techniques have been adopted to improve NIRST system performance, which can facilitate the use of NIRST in breast cancer detection, diagnosis, and treatment. The purpose of this study is to review the current progress on NIRST systems and summarize their advantages and limitations. We also report recent clinical applications of NIRST systems in breast imaging and discuss the challenges and future developments.ProgressThis paper presents a review of imaging types (Fig. 2) involved in data acquisition. First, continuous wave (CW) systems, including available commercial instruments, are introduced (Fig. 3). The widely used frequency-domain (FD) and time-domain (TD) systems are summarized (Figs. 4 and 5). The emerging hybrid imaging types and relevant prototype systems are also reviewed (Fig. 6). The integration of conventional breast cancer-imaging systems into NIRST can enhance spatial resolution of NIRST and improve lesion characterization. Therefore, the multimodality imaging systems widely used in breast imaging are also reported (Figs. 7 and 8), particularly in magnetic resonance imaging (MRI)/NIRST interfaces. As incorporating structural information is critical for the accurate clinical diagnosis of breast cancer, the methods including hard prior, soft prior, direct regularization imaging, and the new deep learning methods are discussed (Fig. 9). Their applications in breast cancer diagnosis and prediction response to breast cancer neoadjuvant chemotherapy are also demonstrated (Figs. 10 and 11).Conclusions and ProspectsNear-infrared spectral tomography can provide functional information regarding breast tissue and be used as a supplemental imaging tool for clinical breast cancer-imaging modalities. However, the primary restriction of NIRST is poor spatial resolution. Recent developments in hybrid imaging types and multimodality imaging have facilitated studies on breast cancer management. In addition, deep learning has been applied to NIRST to improve lesion characterization and reduce computational time. The proposed method is expected to assist in breast diagnosis.
SignificanceFor over half a century, the three main pillars of conventional cancer therapy are surgery, chemotherapy, and radiotherapy. However, these treatment methods have inherent limitations, as they inevitably cause severe damage to normal cells, particularly immune cells. The discovery and development of immunotherapy show promising clinical applications. Nonetheless, immunotherapy is a double-edged sword, often leading to the occurrence of immune-related adverse events (irAEs) because of off-target effects. Therefore, the current focus in cancer research is to explore treatment strategies that can activate local immune responses while enhancing tumor specificity.Near-infrared photoimmunotherapy (NIR-PIT) is a novel tumor therapy, and it depends on a single antibody-photo absorber conjugate (APC), which combines a monoclonal antibody (McAb) targeted on tumor features with IRDye700DX (IR700). Except for its specific antitumor mechanisms, a unique aspect of NIR-PIT is its direct impact on blood drug delivery. The super-enhanced permeability and retention (SUPR) effects facilitate the rapid leakage of drugs into the tumor, favoring the induction of cytotoxic effects. However, the presence of the “binding site barrier” indicates that using antibodies with low affinity or targeting antibodies with low antigen expression may promote a more even distribution of APCs within the tumor parenchyma. In recent years, researchers have investigated the use of different targeting segments in NIR-PIT, enhancing the tumor immunogenicity, targeting ability, stability, and flexibility of NIR-PIT drugs. This approach has shown considerable potential for application in various types of tumors, with some related clinical trials yielding satisfactory results.Studies have shown a close association between the suppressive tumor microenvironment (TME) and the growth and progression of cancer. In recent years, the targets of APCs in NIR-PIT have expanded to surface proteins of non-tumor cells in TME. The combination therapy of NIR-PIT with immune checkpoint blockade (ICB) has also shown promising experimental results. The development and continuous improvement of optical devices also facilitate the monitoring and evaluation of the therapeutic effects of NIR-PIT. Therefore, it is necessary to summarize previous relevant research to provide a rational reference for the clinical research and application of NIR-PIT.ProgressThe primary mechanism through which NIR-PIT exerts its cytotoxic effects is via a photochemical reaction from IR700. Under near-infrared light, IR700 in APCs undergoes a photocatalytic transformation, changing its chemical properties from hydrophilic to hydrophobic, and aggregating in an aqueous solution. This process leads to the denaturation of the cell membrane antigens bound to it, physical damage to the cell membrane and cell rupture, increased transmembrane water flow, and cell death (Fig.1). Simultaneously, the rapid release of tumor-associated antigens (TAAs) and damage-associated molecular patterns (DAMPs) during NIR-PIT induces immunogenic cell death (ICD), and subsequently, activates the antitumor immune response of the host, enhancing the activation of systemic immune responses to attack other cancer cells and further amplifying the therapeutic effects of NIR-PIT (Fig.2). Current NIR-PIT treatment strategies targeting key components in TME, including immune inhibitory cells (Tregs and MDSCs), cancer-associated fibroblasts (CAFs), and blood vessels, are listed in Table 1. The design principles of APCs and relevant experimental results are also presented. Subsequently, the combined therapeutic strategies and efficacy of immune checkpoint inhibitors with NIR-PIT targeting different cell surface proteins are elucidated. Given the heterogeneity of immune cell populations in different tumors, the choice of ICBs can be based on the expression levels of specific immune checkpoint molecules in their respective tumors.In addition, the progress of clinical trials related to NIR-PIT is summarized, demonstrating that cetuximab-IR700 (RM-1929) can elicit effective antitumor responses in patients with locally recurrent HNSCC where conventional clinical treatments are less effective. Furthermore, the SUPR effect can be quantified using indocyanine green (ICG)-fluorescence and magnetic resonance imaging (MRI) contrast agents to monitor and identify the viability of NIR-PIT. 18F-fluorode-oxyglucose positron emission tomography (18F-FDG-PET), fluorescence lifetime imaging, and bioluminescence imaging can evaluate acute NIR-PIT treatment in preclinical studies. Moreover, the micro distribution of the NIR-PIT agent and its therapeutic effects is monitored using a two-channel fluorescence fiber-imaging system and two-photon microscopy with and without a microprism. The tumoricidal effects and hemodynamic changes induced by NIR-PIT can be monitored by 13C MRI, blood oxygenation level dependent (BOLD) MRI, and photoacoustic imaging.The current investigation of NIR-PIT is relatively limited. In summary, the limitations of replicating IRdye700-McAb conjugates in NIR-PIT, the penetration and uniformity of near-infrared light irradiation, differences in the types and expression of target molecules in different types of tumors, and the safe range of APC dosage in NIR-PIT still require detailed investigations.Conclusions and ProspectsExtensive in vitro and in vivo models study on NIR-PIT have been conducted for various types of tumors, with promising therapeutic outcomes. With its broad and flexible application scope, and various approaches to enhance its efficacy, NIR-PIT has significant potential as a valuable method for cancer treatment.
ObjectiveCurrently, most commercial optical coherence angiography (OCTA) systems lack a real-time display of en face OCTA images, which makes it difficult for operators to obtain intuitive feedback on data quality and adjust the system quickly and accurately in a single acquisition of OCTA volume data. In the process of dynamic acquisition of OCTA volume data, determining the state changes of the subjects is difficult, resulting in invalid data acquisition. In an experiment on flicker light-induced functional retinal hyperemia, which provides a new perspective for the early screening of human diabetic retinopathy, the continuous collection of multiple groups of three-dimensional data may be invalid because of the poor quality of one group, thereby wasting data processing time. Therefore, a real-time display of the experimental results is required. Although GPU-based OCTA data real-time processing methods have been proposed, the speed of the existing real-time processing methods still needs to be improved to adapt to high-speed scanning OCTA systems.MethodsIt is developed on a spectral-domain OCT (SD-OCT) system. Limited by the frame grabber, the maximum acquisition line speed of the system was 120 kHz in the high-bit-depth mode and 250 kHz in the low-bit-depth mode. An optical coherence angiography algorithm based on the inverse signal-to-noise ratio (SNR) and complex-valued decorrelation (ID-OCTA) was used to extract blood signals by adaptive SNR and achieve high-quality angiography. The sum of absolute differences (SAD) algorithm was used to register OCT images, and the retinal OCT images were segmented by a vertical gradient distribution, which is convenient for fast parallel processing on a Graphics Processing Unit (GPU). This study proposes a real-time processing framework based on a GPU (Fig.1), which uses texture memory to realize fast interpolation and filtering calculations and the CUDA stream to mask the time delay of data transmission between the host and GPU. We developed a real-time processing program using C++ and CUDA and a multithread system control program using the C++ and MFC libraries. To compare the guiding effect of the real-time data processing method in this study and the method using only a CPU, two real-time display modes were used for data acquisition: en face OCTA images and cross-sectional OCT images. Moderately experienced operators collected multiple groups of data in these modes within 40 s. Three sets of data were collected continuously in 12 s to simulate the dynamic acquisition of OCTA volume data. The quality of the collected data was evaluated using the en face OCTA image quality index. In the flicker light-induced functional retinal hyperemia experiment in mice, the experimental success criteria and quantification parameters were set. Operators conducted multiple experiments to compare the experimental success rates of the two real-time display modes.Results and DiscussionsThe en face OCTA image real-time display was realized in the system with a 250 kHz line scanning speed (Fig.2), and the line-processing rate was 365 kHz (Table 1). Compared with the real-time display of the cross-sectional OCT image, the real-time en face OCTA image can guide system refraction and eye position adjustment more accurately and quickly (Fig.3, Table 2). In dynamic OCTA acquisition, the real-time display of en face OCTA images can reflect the movement of the mouse eye and its jitter, which is not evident in cross-sectional OCT images (Fig.4). In an experiment on functional retinal hyperemia, the real-time display video generated immediately after the experiment (Fig.6) can be used as a preview of the experimental results. Compared with 66.7% in the cross-sectional OCT image real-time display mode, the experimental success rate of the en face OCTA image real-time display mode was 93.3%, which proves that this mode helps avoid the situation where system adjustment and subject status problems lead to experimental failure (Table 3, Fig.5). The system can help the experimenter screen unqualified data and quickly judge the experimental results. In the future, the system could replace the frame grabber that supports a higher acquisition speed to improve its scanning speed.ConclusionsWe realized the real-time display of en face OCTA images in a 250 kHz SD-OCT system. Compared to the real-time display of cross-sectional OCT images using a CPU, the real-time display method in this study helps the operator adjust the system more quickly and accurately during the single acquisition of OCTA volume data and provides feedback on the subject eye state during the dynamic acquisition of OCTA volume data. The proposed real-time display method was confirmed to have a data quality feedback function in the experiment of flicker light-induced functional retinal hyperemia, which improved the experimental success rate. The line processing rate reaches 365 kHz, which can be adapted to a high-speed scanning OCTA system.
ObjectiveRespiratory viruses possess strong infectivity, rapid transmission, short incubation periods, and sudden onset of illness. These features have led to widespread global transmission, significantly affecting the health of children worldwide. In addition, these viruses have caused significant economic losses and casualties in various countries. Antibiotics are commonly used to control respiratory infections in humans. Therefore, accurate and rapid understanding of the course of respiratory infections is the foundation for selecting a treatment plan.In biomedical imaging, various imaging methods can reveal microscopic and macroscopic phenomena within organisms. These methods include magnetic resonance imaging (MRI), computed tomography, positron emission tomography, ultrasound (US) imaging, optical coherence tomography, and fluorescence imaging. These technologies provide rich information, thereby contributing to a comprehensive understanding of the characteristics of respiratory infections and supporting the development of rational treatment plans. Owing to limitations in specificity, resolution, and radiation, these imaging techniques lack the ability to accurately image biological structures in the early stages of disease development. In this study, the noninvasive, deep-penetrating, and high spatial resolution advantages of photoacoustic (PA) imaging (PAI) are utilized. This is combined with the excellent fluorescence properties of the exogenous contrast agent indocyanine green nanoparticles (nano-ICG) in the near-infrared region and the high expression of macrophages during inflammation. This combination enables the visualization of the development of respiratory inflammation.Through the establishment of animal models and in vivo experiments, we quantitatively evaluate the macrophage expression in acute respiratory infections, as shown in Fig.1. Research on PAI is expected to provide a new approach for the noninvasive quantitative assessment of inflammation in acute respiratory infections.MethodsThis study uses a respiratory inflammation mouse model for photoacoustic imaging. Initially, the mice are anesthetized using isoflurane with volume fraction of 1.5%, followed by the instillation of lipopolysaccharide (LPS) solution into the mouse respiratory tract to construct the respiratory inflammation model group after two days. Five mice are selected from the Control and Model groups for further studies. Subsequently, the ultraviolet absorption spectra and cytotoxicity of nano-ICG materials are studied under irradiation at different wavelengths. The internalization dynamics of macrophages after nano-ICG injection are investigated. Finally, a PA-US dual-mode small animal imaging system is used to image different groups (Control and Model groups). Imaging is conducted before nano-ICG instillation and when the post-injection time is 15, 30, and 60 min in each group of mice. PA and US data collected from the experiment are subjected to offline quantitative analysis using Vevo Lab Software 3.2.0 to observe the overall respiratory inflammation under PAI.Results and DiscussionsTransmission electron microscopy is used to characterize the shape and size of the exogenous contrast agent, nano-ICG. As shown in Fig.2 (a), Nano-ICG has an average size of approximately 65 nm with a round shape and aggregated distribution. Subsequently, the cell counting kit is employed to evaluate the in vitro viability of macrophages, and the absorbance of each well is determined using enzyme-linked immunosorbent assay, as shown in Figs.2(b) and 2(b). The internalization of nano-ICG at different time points after injection is observed using confocal fluorescence microscope, as shown in Fig.3. These results indicate that nano-ICG continue to be internalized by the macrophages within one hour after injection. Additionally, laser confocal microscope images exhibit a positive correlation between the uptake of nano-ICG by macrophages and time. After engulfing the nanoparticles, the imaging effect of macrophages becomes more prominent. Within the first 15 min after nano-ICG injection in mice, Model group exhibits an enhanced trend in the PA signal compared with the normal group. In Control group, the PA signal of nano-ICG exhibits a decreasing trend over time, whereas in Model group, the corresponding PA signal continues to increase. After 30 min, the PAI images of Control and Model groups exhibit more noticeable contrast. After 60 min, Model group exhibits the strongest PA signal, showing a more significant contrast than Control group, as shown in Fig.4(a). In Control group, the amount of nano-ICG in the mouse airways continuously decreases with increasing post-injection time, as shown in Fig.4(b). In Model group, the quantity of nano-ICG on the mouse airway wall increases continuously with the post-injection time, as shown in Fig.4(c). These results indicate that nano-ICG can effectively reflect the degree of development of inflammatory cells on the respiratory wall when the post-injection time is 60 min. Three-dimensional PAI images of respiratory inflammation provide more accurate information on respiratory wall inflammation, as shown in Fig.5(a). The coronal images generated by two-dimensional PAI scans, indicate the presence of inflammatory cell aggregation in the respiratory tract at that position. Figure 5(b) validates the accuracy of three-dimensional PAI images by showing images of inflammatory and non-inflammatory cells in the respiratory tract using an in vivo imaging system (IVIS) for small animals. Although PAI can visually present respiratory inflammation, some mice must be euthanized for pathological sectioning and staining to gain a more comprehensive understanding of the morphological and structural changes in inflammation. Histological results are shown in Fig.6. Control group sections exhibit a light pink color in the airways with no thickening on the inner side of the tube wall and smooth and regular surfaces without apparent lesions. In contrast, Model group sections exhibit noticeable bleeding, significant swelling, scattered bleeding points on the surface, infiltration of inflammatory cells on the inner side of the tube wall, and increased secretion into the lumen, consistent with the imaging structures of PAI.ConclusionsThis study successfully establishes a mouse model for acute respiratory inflammation and utilizes nano-ICG to observe respiratory inflammation, confirming the feasibility of evaluating inflammation using PAI. The PAI results for inflammation in the model are consistent with the pathological and IVIS results. This research provides new methods and insights for assessing respiratory inflammation. In summary, PAI is widely applicable to respiratory inflammation research because of its unique imaging capabilities, non-invasiveness, and high resolution. This study provides strong support for a deeper understanding of the development of respiratory inflammation and evaluation of treatment effectiveness.
ObjectiveNucleic acid detection methods enable the rapid identification of specific genetic indicators. However, their extensive application is limited by the sequential use of multiple instruments and the high technical requirements for operators. A fully integrated nucleic acid analysis system can automate the sample-to-answer detection process. However, nucleic acid amplification via PCR technology is time-consuming, and stringent requirements are placed on temperature control accuracy and heating/cooling speed, which increases the manufacturing cost of the corresponding instruments. These systems also lack the flexibility to accommodate variations in sample preprocessing methods and nucleic acid extraction protocols for different types of clinical samples. To address these challenges, we present a novel, fully integrated nucleic acid analysis system that contains a syringe-based sample processing and nucleic acid extraction module, a fully enclosed PC-based multiplexed detection microfluidic chip, and a nucleic acid amplification product detection module. We applied this system for the precise medical testing of various pathogenic infections in gynecology. The results indicate that our system can achieve precise medical molecular diagnosis with the fully integrated automation of a micro-nano reaction system, as well as speedy processing, high sensitivity and specificity, and multiplexed parallel detection. The system also shows potential applications in the in vitro identification of genetic and other nucleic acid-related diseases, holding significant social and economic value for the prevention and control of major diseases in China.MethodsThis paper presents a fully integrated nucleic acid analysis system based on a syringe microfluidic chip. The system consists of two modules. The first is an automated syringe nucleic acid extraction module that accommodates multiple nucleic acid extraction techniques based on different clinical sample types. This process involves four primary steps: sample loading, incubation, washing, and lysis by heating and shaking. The second module is an isothermally amplified nucleic acid detection module capable of simultaneous multivariate detection. The nucleic acid amplification and detection platform, based on microfluidic technology, includes a disk-based microfluidic chip that serves as a nucleic acid isothermal amplification carrier, a temperature control platform ensuring precise isothermal amplification of nucleic acids, a fluorescence detection platform for real-time monitoring of nucleic acid amplification, and a software analysis platform for controlling and analyzing the entire system. The two modules perform their respective functions independently and can also be combined into an integrated syringe-microfluidic chip nucleic acid analysis system, forming a fully integrated micro-nano detection platform that rapidly automates both nucleic acid extraction and detection and is capable of detecting multiple indicators in parallel.Results and DiscussionsThe fully integrated system was evaluated using a standard Candida tropical culture strain and 64 clinical swab samples obtained from patients with vulvovaginal candidiasis. The results show that the detection concentration threshold of 3.95×102 CFU/mL was comparable to that of the conventional nucleic acid detection method, which involves extracting nucleic acid with a reagent kit and amplifying it on a commercial PCR machine. The sample preparation is more convenient and fast, with only one sample loading step, and nucleic acid extraction takes 10 min, resulting in significant time and labor savings (Table 1). A chi-square value of 1 and a Kappa value of 0.950 indicate a strong correlation with the gold standard method of clinical microbial culture (Table 2). Although a false-negative result with two samples containing Candida albicans was obtained, optimizing the primer sequences and reaction systems for isothermal amplification can address this problem. Therefore, this comprehensive integrated nucleic acid detection platform provides rapid and accurate multiplexed detection for the diagnosis of clinical microbial pathogen infections and has advanced technological capabilities.ConclusionsIn this study, a fully integrated nucleic acid analysis system based on a syringe microfluidic chip is developed, which consists of two modules. One is an automatic syringe nucleic acid extraction module, and the other is an isothermally amplified nucleic acid detection module based on a microfluidic chip. The system was tested using a standard Candida tropical culture strain and 64 clinical swab samples of vulvovaginal candidiasis. The results show that the minimum detection concentration of the bacterial solution in the system is 3.95×102 CFU/mL, and a more convenient and rapid sample preparation is achieved with only one sample loading step with a nucleic acid extraction duration of 10 min. Compared with the gold standard culture method, a chi-square test and Kappa value of 1 and 0.950, respectively, indicate that there is no significant difference between the two methods, with high consistency. The syringe-microfluidic chip-based, fully integrated nucleic acid analysis system detailed in this study provides a reliable platform for the rapid detection of nucleic acids using multiple indicators in a micro-nano system. The system offers precise detection and convenient analysis and supports applications in clinical medicine, grassroots screening for infectious diseases, health prevention programs, and basic biomedical research. This underscores the significance of this system in molecular diagnostics with in vitro precision in clinical settings, such as genetic disease identification and tumor molecular marker co-detection, as well as its potential uses in food safety and preventive healthcare applications.
ObjectiveVascular imaging is crucial for medical diagnosis, and it facilitates disease diagnosis, disease progression monitoring, surgical planning and navigation, and patient prognosis evaluation. However, conventional imaging methods encounter challenges in rapid and noninvasively imaging of superficial blood vessels because of the similarities in texture and color with surrounding tissues. In clinical surgery, preoperative superficial vascular imaging and intraoperative vascular display are beneficial for reducing bleeding, facilitating surgical navigation and path planning, and promoting postoperative blood supply recovery.MethodsA fluorescent imaging system in the second near-infrared window (NIR-II) to capture images of the forearm vascular network in patients was used in this study. The research involved 12 patients, with indocyanine green serving as a contrast agent for obtaining NIR-II images of the forearm blood vessels. These images were used to observe the anatomical structure of the venous vascular network. Furthermore, computer-aided analysis was employed to improve image quality, support surgical path planning and prognosis prediction, and provide valuable guidance for clinical practitioners.Results and DiscussionsHigh-resolution and high-contrast images are captured using NIR-II fluorescence angiography. The clarity of these images are enhanced using artificial intelligence techniques. Computer simulation was employed to simulate venous network reflux. The agreement between the measurement results obtained from this technique and ultrasound was evaluated using the Bland Altman plot and consistency measurements.ConclusionsThe acquired images are used to examine the anatomical structure of the venous vascular network and combined with blood flow simulation. This integration aims to assist clinicians in determining optimal surgical trajectories and predicting outcomes. The application of NIR-II fluorescence imaging and computational simulation techniques has potential in providing valuable support for surgeons in performing various vascular procedures in the future.
ObjectiveThe structural characteristics of biological tissues can provide essential information for diagnosing clinical diseases. Medical imaging methods, such as X-ray imaging, computed tomography, magnetic resonance imaging, positron emission tomography, and ultrasound imaging, can obtain the structure and function of the tissues; however, these methods cannot detect small lesions due to low imaging resolutions. A biopsy, the gold standard for tumor diagnosis, is painful and invasive, and some tissues cannot be sampled. Optical coherence tomography (OCT) is a label-free, noninvasive, three-dimensional optical imaging method with micrometer resolution and is used for optical biopsy. In the traditional benchtop OCT system, the large scanning probe fixed on a bench cannot reach into a narrow cavity, and the detection process requires a high degree of patient cooperation. Therefore, the use of benchtop OCT systems for clinical applications is limited to a certain extent. A handheld OCT system has a separated sample arm packaged into a miniaturized handheld probe, which is connected to the main OCT system via an optical fiber. The miniaturized probe can be held conveniently and inserted into the narrow cavity, increasing the applicability and flexibility. We propose a video-guided handheld high-speed OCT system with an A-line speed of 200 kHz. The compact handheld probe is easy to hold and can be inserted into narrow cavities. A camera integrated into the probe can capture real-time video for guiding OCT imaging. An image registration method is also developed to eliminate image misalignment due to hand tremors during OCT imaging.MethodsA handheld OCT system based on a swept source was built for tissue imaging, as shown in Figure 1. The handheld probe was connected to the main system through an optical fiber. The handheld probe was made to have a smaller size and lower power consumption by employing a microelectromechanical system-based scanner for beam scanning. A visible imaging camera integrated inside the handheld probe allows for real-time imaging, facilitating rapid localization of the region of interest, and guiding OCT imaging. The system has a high scanning speed with an A-line rate of 200 kHz, a lateral resolution of 31.4 μm, and an axial resolution of 5.2 μm in tissue. To improve the image quality, an image registration method was developed to eliminate image dithering. The handheld OCT system was validated using ex-vivo porcine cornea and tooth. The images obtained by the handheld OCT system were also compared with those obtained by the benchtop OCT system.Results and DiscussionsThe ex-vivo porcine cornea and tooth were imaged using the handheld OCT system, as shown in Figure 2. Figures 2(a) and 2(d) show the images of the cornea and tooth, respectively, captured by a cell phone. Real-time videos can be captured to guide the imaging location and determine the region of interest using the camera in the handheld OCT system. The images of the cornea and tooth captured by the video camera are shown in Figures 2(b) and 2(e), respectively. Single B-scan images of the cornea and tooth are captured by the handheld OCT system, as shown in Figures 2(c) and 2(f), respectively. The results show that the handheld OCT system can acquire high-resolution cross-sectional structural images for the cornea and tooth. During imaging using the handheld probe, the hand tremor causes OCT image misalignment, and image registration is required. Figure 3 shows the OCT images of the porcine cornea and tooth with/without image registration. After multiple rounds of B-scanning at the same location, the images were averaged, as shown in Figures 3(a) and 3(c). The averaged images are blurry, showing image misalignment. After image registration, the image misalignment is corrected, and the averaging B-scan images present a clear tissue structure, as shown in Figures 3(b) and 3(d). To evaluate the imaging performance, the images obtained from the handheld OCT system were compared with those from the benchtop OCT system, as shown in Figure 4. Figures 4(a) and 4(b) show the single B-scan images of the ex-vivo porcine tooth from the benchtop and handheld OCT systems, respectively. The results show that there are no significant differences between the images acquired by the two systems. The CNRs of the images from the handheld and benchtop OCT systems are 3.28±0.01 and 3.30±0.02, respectively. As there is no image misalignment during imaging using the benchtop OCT system, it can provide a reference for evaluating the image registration method. After image registration, the averaging B-scan images from the handheld OCT system show a structure similar to that of the images from the benchtop OCT system. Moreover, the registered images from the handheld OCT system have a quality similar to that of the images from the benchtop OCT system.ConclusionsIn this study, a video-guided high-speed handheld OCT system with an A-line scanning rate of 200 kHz is designed and constructed. Compared with the traditional benchtop OCT system, the handheld system has a compact and easy-to-hold handheld probe, which extends the applications and increases the flexibility of OCT imaging. A video camera inside the probe allows real-time imaging to quickly localize the region of interest and guide the OCT image. An image registration method can eliminate image misalignment during OCT imaging. The imaging performance of the system was verified by imaging ex-vivo porcine cornea and tooth. The results show that the handheld OCT system can provide a more convenient method for tissue imaging, thus exhibiting great potential for imaging the tissues in a narrow cavity and serving the needs of less-cooperative patients.
ObjectiveWearable flexible electronics is one of the development trends in medical-health monitoring, particularly cardiovascular-disease monitoring. Pulse wave is an important source of information for assessing cardiovascular health; however, it is a non-stationary weak signal and imposes high requirements on the sensitivity and stability of detection. To solve the key technical problems of wearable health monitoring, a graphene-based flexible pressure sensor with a multilevel branched microstructure is designed and developed in this study, which significantly improves the sensing performance of pulse waves and forms the foundation for a wearable flexible pressure sensor. The sensing health-monitoring system is developed using a sensing-like cuffless blood-pressure-monitoring algorithm based on single-point radial artery pulse waves. The prediction errors of the system for human systolic blood pressure (SBP) and diastolic blood pressure (DBP) are (0.7±10.5) mmHg and (0.5±6.1) mmHg, respectively. The findings of this study can provide important technical support for cardiovascular health-monitoring systems and application research, as well as for wearable precision medical-health monitoring.MethodsFlexible pressure sensors based on array structures exhibit key technical issues such as difficulty in achieving high sensitivity and a wide pressure-detection range, as well as limited usage. Hence, a hierarchical branch (HB) structure pressure-sensor design scheme is proposed in this study to improve the performance of array-microstructure pressure sensors. First, we completed the design of the HB structure via finite-element analysis. Results of the finite-element analysis reveal the unique effect of the HB structure: it not only includes the elastic-modulus- reduction effect caused by the superposition of multiple elastic layers but also integrates the pressure-diffusion effect caused by the HB structure, thus realizing the gradual activation and further strengthening of the active-layer conduction path. As such, the deformation range of the elastic layer (sensor pressure-detection range) and the deformation sensitivity to pressure (sensor sensitivity) can be improved. To solve the problem wherein the existing blood-pressure-detection equipment requires cuff pressurization and continuous blood-pressure monitoring cannot be achieved easily without pressurization interference, we construct a cuffless blood-pressure detection model, the class-aware model based on the Moens-Korteweg (M-K) equation and Transformers (the CAMKformer model). This model incorporates the idea of ??cascade learning, uses the basic formula for blood-pressure calculation based on pulse-wave conduction velocity as the principle, and applies the Transformer model to classify the input pulse wave for blood pressure, thus forming a two-stage cuffless blood-pressure detection model. Compared with conventional machine-learning algorithms based only on formulas or tree models, this model combines formula-related features with original pulse-wave data, where the complex feature-extraction capabilities of deep-learning models and the strong interpretability of theoretical models are fully utilized. In addition to affording high robustness, it integrates multimodal blood-pressure related information (discrete pulse-wave characteristics and continuous pulse-wave data), thus significantly reducing the blood-pressure detection errors inherent in conventional research methods.Results and DiscussionsExperimental results show that the HB structure enables flexible pressure sensors based on array microstructures to simultaneously improve sensitivity (an increase by 14 times, which is more significant than that of previously published single-layer structure strategies) and the linear range (Fig.6). Additionally, the HB structural strategy based on template and multilayer superposition methods offers significant advantages in terms of structural uniformity, adjustability, and scalability. For example, molds can be fabricated via highly controllable processes (such as photolithography), thus allowing parameters such as structural shape and size to be adjusted. We believe that the HB strategy can be used as a general strategy to adjust mechanical-stress transfer and optimize sensor performance, as well as exhibits broad application prospects in sensor design. A diverse database can further demonstrate the robustness and generalizability of the CAMKformer model. The results show that the wearable system and CAMKformer model constructed in this study can adapt promptly to the pulse-wave characteristics of different individuals and accurately detect human SBP and DBP [with errors of (0.7±10.5) mmHg and (0.5±6.1) mmHg, respectively, as shown in Table 3]. Different from the pressurized blood-pressure monitoring method of conventional electronic sphygmomanometers and commercial blood-pressure measurement smart watches, the abovementioned system does not require pressure application to the user’s radial artery to detect blood pressure; hence, it is suitable for continuously measuring the user’s blood pressure during daily activities or at night, as blood pressure changes during sleep. In addition, this model uses a single-cycle pulse wave as input, presents a simple system configuration, and is highly flexible for use.ConclusionsIn this study, wearable flexible electronic technology and medical-health monitoring are adopted as the research background. The requirements of wearable cardiovascular health monitoring are identified, and the associated principles, devices, systems, and application levels are investigated systematically. First, a design scheme for a HB structure flexible pressure sensor is proposed, and a graphene HB structure flexible pressure sensor is reconstructed simultaneously with a pulse-wave measurement system. Experimental results show that the bionic HB structure strategy enhances the sensitivity (an increase by 14 times) and linear range (4.6 times expansion) of the array-based microstructure pressure sensor, thus enabling distortion-free and accurate measurements of pulse waves. Subsequently, a class-aware cuffless blood-pressure-detection model is established. This model, which is based on the abovementioned flexible pressure sensor, obtains single-point pulse waves of the radial artery. Additionally, it uses a deep-learning model based on the Transformer for blood-pressure classification and a theoretical model based on the M-K equation for blood-pressure prediction. Compared with conventional machine-learning algorithms based only on formulas or tree models, this algorithm combines commonly used pulse features with original pulse-wave data, where the complex feature-extraction capabilities of deep-learning models and the strong interpretability and robustness of theoretical models are fully exploited. Owing to its high stickiness, it realizes the fusion of multimodal pulse-related information and significantly reduces the error of cuffless blood-pressure detection [the errors are (0.7±10.5) mmHg and (0.5±6.1) mmHg for SBP and DBP, respectively], thus satisfying the international standards for non-invasive blood-pressure monitors. The cuffless blood-pressure monitoring system proposed herein is devoid of external pressure interference. Additionally, it is expected to transform the blood-pressure dynamic detection mode from “single-point, high-dispersion transient detection” to “multipoint, low-dispersion online monitoring,” thus facilitating users or doctors in dynamically monitoring blood-pressure changes to achieve early proactive screening of hypertension and improve hypertension early-warning and monitoring capabilities.
ObjectiveThe rupture of vulnerable plaques caused by atherosclerosis has become one of the most serious threats to human health. Intravascular optical coherence tomography (IVOCT) can accurately identify vulnerable plaque characteristics, such as thin-cap fibroatheroma plaques, owing to its high resolution, and has gradually become the gold standard for the diagnosis of vulnerable plaques. Typically, clinicians must manually mark the location of plaques in an image based on their experience. However, this method is time-consuming and labor-intensive and is susceptible to the subjective assessment of the clinician. Manual interpretation significantly reduces the speed and precision of vulnerable plaque diagnosis. Some studies based on traditional machine learning have been conducted for the detection of vulnerable plaques and have achieved the classification of single-frame images. However, the accuracy of frame-level information is insufficient to assist clinicians in determining treatment strategies. These methods require a second interpretation by clinicians. This study proposes an evaluation algorithm for vulnerable plaque identification in IVOCT images based on an improved Faster R-CNN (regional convolutional neural network) framework. In addition to accurately locating vulnerable plaques, the algorithm can quantitatively assess the risk of plaque rupture, providing diagnostic suggestions to clinicians and assisting in the formulation of treatment plans. The comprehensive nature of this approach is expected to play an important role in improving the efficiency and precision of vulnerable plaque diagnosis.Methods This study is divided into two partsautomatic identification of vulnerable plaques and assessment of vulnerable plaque rupture risk. To identify vulnerable plaques based on the Faster R-CNN, this study proposes an improved strategy for enhanced cyclic shift data, (X, W) encoding BBox, and the introduction of additional semantic segmentation heads according to the characteristics of IVOCT images. The network is generally divided into four parts (feature extraction, region extraction, secondary detection, and A-scan classification), allowing the network to locate vulnerable plaques with higher accuracy. In this study, the angle of accumulation of the lesion, the thickness of the fibrous cap, macrophage infiltration, superficial microcalcification, and vascular stenosis degree of vulnerable plaques are selected as indicators to assess the risk of rupture. The vascular lumen area is used to characterize the degree of vascular stenosis in vulnerable plaques; the smaller the lumen area, the more severe the stenosis. Furthermore, an adaptive threshold method is designed to calculate the thickness of the fibrous cap, which is considered thin when the thickness is less than 65 μm. The risk of plaque rupture is indicated by a lesion accumulation angle greater than 90°, and a polar graph is used to measure the lesion accumulation angle. To identify superficial microcalcifications and macrophage infiltration, features are extracted from the images and reclassified. The application of these methods makes our study more comprehensive and accurate.Results and DiscussionsThe proposed method is trained and tested using the public dataset CCCV2017 IVOCT. This study presents the results of the ablation experiment for Faster R-CNN (Table 2). The improved network performs well in positioning vulnerable plaques, with mAP50 increasing to 0.744 and the Dice value increasing to 0.905. Compared with weakly supervised detection (WSD) and salient-region-based convolutional neural network (SRCNN) methods, the method proposed in this study significantly improves the recall and Dice values (Table 3). The intersection of union (IOU) value of the lumen area is 0.9445, and the prediction result is consistent with actual result of the lumen area [Fig.7(c)]. The root mean square error RMSE and the goodness of fit R2 are used to verify the feasibility of the calculation of the thickness of the fiber cap, and the test results are 1.17 pixel and 0.62, respectively. After positioning the region accurately, the cumulative angle of the lesion is also accurately assessed [Fig.7(d)]. To evaluate the performance of the model in predicting superficial plaque microcalcifications and macrophage infiltration, a comprehensive analysis is performed using a confusion matrix [Figs.7(e) and (f)]. These results demonstrate that the proposed method achieves satisfactory results for multiple evaluation metrics and provides a reliable solution for the identification of vulnerable plaques and rupture risk assessment.ConclusionsIn this study, the cyclic shift, (X, W), and encoding BBox are added, and additional semantic segmentation heads are introduced to the Faster R-CNN network to improve the detection performance for vulnerable plaques. Compared to the initial network and adding only a single change, the method proposed in this study significantly improves the mAP50 and Dice values of the network. Compared with WSD and SRCNN, our method also achieves significant improvements in the recall rate and Dice value. Furthermore, to obtain accurate location results for the vulnerable plaque region, the angle of the plaque region is used to measure the angle of accumulation of the lesion, and the cumulative pixels of the fiber cap are used to calculate the thickness of the fiber cap. Deep neural network features combined with gradient direction histogram features are used to analyze macrophage infiltration and superficial microcalcification, and the vascular stenosis degree is evaluated in the lesion lumen region. Multiple single-evaluation results are used to measure the risk of rupture of vulnerable plaques. The comprehensive method proposed in this paper achieves a significant breakthrough in vulnerable plaque detection and provides more comprehensive and reliable data support for clinical diagnosis in terms of rupture risk assessment.
ObjectiveLong-term cellular imaging and analysis are pivotal for biomedical research and clinical diagnosis. Effective and continuous nanoscale imaging of living cells reveals natural, dynamic, and subcellular alterations at the microscale, which are crucial for understanding long-term cellular morphology and metabolism. Interferometric spectroscopy, which has garnered considerable attention for molecular detection, elucidates nanoscale fluctuations on the sample surfaces. This technology dispenses with the need for precise instruments for generating analytically coherent signals or costly optical setups. The algorithmic reconstruction of the collected interferometric spectral data yields quantitative imaging outcomes. Furthermore, it obviates the reliance on the lens selection ability of the microscope objective, thereby allowing space for the integration of microcell culture devices. Consequently, interferometric spectroscopy analysis has emerged as a highly promising method for addressing the extant challenges in the in situ analysis of unlabeled live cells. A hyperspectral interferometric imaging system for long-term in situ analysis of unlabeled live cells is introduced in this paper, facilitating the quantitative imaging of nanostructures and measurements of the dry mass. Moreover, the system is employed in investigating the quantitative nanostructure and dry mass dynamics of various cells throughout the entire cell cycle, which showcases a potential application of the proposed method and system in the biomedical realm.MethodsThe coherent signals generated by light reflected from a substrate and the scattered light within a cell provide valuable insights into the three-dimensional structure and dry mass distribution of the sample. A pivotal innovation in this study is the proposal for a label-free quantitative microscopy imaging technique for hyperspectral interferometric reconstruction. By formulating a mathematical model to characterize the interferometric signal, a sample quantitative reconstruction algorithm was devised, enabling the acquisition of quantitative nanostructures and the dry mass distribution of live cells. In the methodology section, we first establish a hyperspectral interference model for adherent cells on silicon wafers, and subsequently propose a label-free quantitative imaging approach based on this model. The phase distribution in the spatial domain, derived from the reflection spectrum containing the interference information, was converted into refractive index and dry mass information. Subsequently, a microscopic imaging system for hyperspectral interference is introduced. Unlike conventional wide-field optical microscopes, this system features a fiber-optic spectrometer structure for hyperspectral imaging. Additionally, a two-dimensional scanning platform with sub-nanometer-level step accuracy was positioned beneath the sample, facilitating point-by-point scanning with a minimum step of 0.16 nm. An integrated live-cell culture incubator was incorporated into this system. Unlike conventional microscope-equipped incubators, the incubator in this system included a liquid delivery function. Finally, a comprehensive overview of the microscale in situ live cell culture device is provided and the performance of the incubator is demonstrated.Results and DiscussionsMicroscopic imaging and hyperspectral interferometric reconstruction imaging of processed three-dimensional silica photon and individually cultured HeLa cells (Fig.5) are demonstrated in this study. The interferometric reconstruction exhibits a thickness error of merely 1.27 nm, accurately restoring the three-dimensional structure of the phantom. Each pixel achieves a dry mass measurement accuracy of 25.75×10-18 g. Moreover, quantitative imaging of the entire cell cycle of HeLa cells and HCerEpiC cells is conducted with a comparison of the stem mass changes between the two cell types (Fig.6). Subsequently, the nuclear-to-cytoplasmic ratios of the two different cell types throughout the cell cycle are determined. Notably, the nuclear-to-cytoplasmic ratio of HeLa cells increases to 24.45% compared with 16.27% in HCerEpiC cells, indicating a relatively higher proportion of nuclear dry mass. The lateral resolution of the imaging system is 708.1 nm, and the axial resolution can achieve 91.89 nm. The complete spectral data of a single cell can be obtained in approximately 1.5 min.ConclusionsAs the fundamental building blocks of life, cells hold significant importance across various application domains, including in clinical medical diagnosis, life science research, and basic medical investigations. However, high-resolution measurement techniques such as electron microscopy, near-field optics, and stochastic optical reconstruction microscopy, as well as separation and purification methods such as chromatography, mass spectrometry, and spectrophotometry, are not conducive to in situ real-time measurements of live cell cultures. The novel hyperspectral white-light interference live-cell microscopy imaging technology proposed in this study captures interferometric hyperspectral data from samples, accomplishes three-dimensional reconstruction using an algorithmic design, and constructs a corresponding automated integrated instrument. This instrument comprises a reflective optical microscopy imaging setup, an interference spectrum acquisition and processing module, a sample nanoscale precision scanning module, and a live cell culture module. To some extent, it addresses the limitations of current measurement techniques and achieves the objective of simultaneously acquiring the quantitative nanostructures and dry mass distribution of live cells. The self-reflective interference structure employed by the system eliminates the need for intricate optical modulation components, exhibiting a straightforward design and convenient operation, thereby introducing a novel imaging approach to the biomedical field.
ObjectiveCorneal laser refractive surgery is a method for correcting vision using lasers to reshape the cornea and change its curvature and thickness. Femtosecond laser corneal cutting is widely used in ophthalmic refractive surgery as a precise, minimally invasive, and controllable surgical technique. In femtosecond laser refractive surgery, the numerical aperture of the optical system determines the focal spot size and required single-pulse energy, which are critical parameters that influence the corneal cutting quality. In this study, we built a femtosecond laser surgery system with an adjustable numerical aperture. We investigated the effect of numerical aperture on cutting quality in the corneal stroma by analyzing the differences in bubble morphology, smoothness of flap separation, and proportion of damaged stromal cells. This study aimed to assist clinicians in selecting the appropriate surgical parameters more effectively.MethodsFreshly enucleated pig eyeballs and New Zealand white rabbits were selected as experimental subjects. By adjusting the diameter of the incident beam, corneal flaps were formed on the pig eyeballs using a femtosecond laser with numerical aperture values of 0.16, 0.30, and 0.80. The morphology of the bubbles after cutting was recorded, and the smoothness of the separation was observed when the corneal flaps were lifted. Cell damage experiments were conducted by cutting New Zealand white rabbit eyeballs with a femtosecond laser at numerical aperture values of 0.16, 0.30, and 0.80. After creating the flap with the femtosecond laser, the rabbit eyeballs were placed in corneal active medium (DX solution) and incubated at 4 ℃ for 6 h to induce apoptosis in the damaged corneal stromal cells. Subsequently, the rabbit eyeballs were removed and prefixed in a 4% paraformaldehyde (PFA) solution for 2 h. After dewaxing and rehydration, the corneal sections were double-stained with DAPI (4,6-diamidino-2-phenylindole, D9542, Sigma-Aldrich) and TUNEL (TdT-mediated dUTP nick end labeling, C1088, Beyotime). Finally, the apoptotic cell counts were determined by imaging the sections under a fluorescence microscope.Results and DiscussionsUnder the three different numerical aperture values (0.16, 0.30, and 0.80), as the numerical aperture increases, the volume of the bubbles decreases gradually, and the density of the bubble layer increases (Figs.5 and 6). This is mainly attributed to the decreasing volume of the focal spot with an increasing numerical aperture, which decreases cavitation bubbles. Corneal flaps formed at a higher numerical aperture are easier to separate. This is primarily because smaller cavitation bubbles result in a denser bubble layers, which facilitates the separation of the interlamellar space with less adhesions between the tissue layers. In the cell damage experiment, as numerical aperture increases, the number of apoptotic cells decreases significantly, as shown in Fig. 7. This is attributed to the decreased single-pulse energy and decreased focal spot size associated with an increase in numerical aperture, which results in smaller photodisruption zones and reduced damage to the surrounding tissues. Therefore, increasing the numerical aperture is beneficial for reducing the extent of stromal cell damage.ConclusionsThe effects of femtosecond laser corneal cutting for different numerical aperture values were investigated experimentally. The morphological differences in cavitation bubbles induced by a femtosecond laser at different numerical aperture values were analyzed, and the ease of separation of the lamellar layers and the extent of cell damage were compared. The results of the experiment show that during femtosecond laser corneal cutting, a higher numerical aperture yields smaller bubbles, denser bubble layers, easier separation of corneal flaps, and lower levels of damage to stromal cells. Therefore, a higher numerical aperture is beneficial in femtosecond laser refractive surgery. Overall, this study provides valuable insights into the effects of numerical aperture on femtosecond laser corneal cutting and highlights the importance of optimizing the numerical aperture to achieve improved treatment outcomes in corneal procedures.
ObjectiveGlioma is an invasive malignant primary brain tumor, characterized by its infiltrative growth pattern, making it challenging to differentiate its boundaries from healthy brain tissues during surgical procedures. Our study aims to address the challenge of accurately distinguishing glioma from healthy brain tissues. Based on a home-made high-resolution polarization-sensitive optical coherence tomography (PS-OCT) system, we conduct ex vivo imaging of normal mouse brain and human glioma model mouse brain. Further, based on the polarization analysis of the tumor and normal brain tissues, we propose a novel tumor differentiation metric called optic axis standard deviation to distinguish normal and glioma tissues. It is proven by the results that high-resolution PS-OCT has great potential in imaging brain tissue and can offer enhanced precision to detect glioma during surgical interventions, which will help to solve the urgent clinical need in neurosurgical practice.MethodsA home-made spectral domain PS-OCT system is constructed by utilizing a superluminescent diode as a broad-spectrum light source. The system is performed with an axial resolution of 3.4 μm in air and a transverse resolution of 4 μm in the focal plane. The low-coherence light beam emitted by the broad-spectrum light source is linearly polarized in the vertical direction after passing through a linear polarizer. Through the integration of a quarter-wave plate and a polarizing beam splitter, circularly polarized light enters the sample arm, while orthogonally polarized signals are detected in the detection arm. After collecting the spectral signals corresponding to the two orthogonal polarization states, the parameters of intensity, phase retardation, and optic axis are calculated and the corresponding OCT images are obtained.Given the similarity of genomes between the mouse and human, the mouse brain is chosen as the imaging object in this study. A human glioma mouse model is established by injecting U87-GFP human glioma cells into the mouse brain. The model is euthanized and fixed 5 weeks after establishment. Subsequently, green fluorescent protein (GFP) fluorescence imaging and PS-OCT imaging are performed on the mouse brain. Three-dimensional field of view of PS-OCT imaging is 6 mm×3 mm×2.3 mm. The three-dimensional image contains 1000 B-scans, and each B-scan image consists of 2000 pixel×2000 pixel (x and z directions). The image in the x-y direction is extracted from the three-dimensional image to obtain the en-face image along the cross section of the sample.Results and DiscussionsIn the OCT images of the normal mouse brain (Figs. 2 and 3), neural fiber bundles in different directions can be clearly observed. In the polarization images, these fiber structures are more pronounced, manifested as radiating short lines. The mouse cerebral cortex exhibits polarization-maintaining properties without birefringence changes. The internal capsule structure between the cortex and internal brain regions is visible with birefringent properties, leading to notable variations in phase retardation. The hippocampal structure lacks birefringent characteristics, resulting in a low value of the phase retardation.Contrasting with fluorescence imaging results to determine the tumor location in the mouse brain (Figs. 4 and 5), it can be seen from the OCT images that there is no significant birefringence change in the tumor tissue, the color of the polarized images becomes more homogenous, and we can find that the infiltration of tumor cells leads to strong fiber damage, and there is no prominent linear structure in the optic axis results.The optic axis histogram (Fig. 6) illustrates distinctive distribution features between normal brain tissue and glioma tissue. The optic axis of normal brain tissue is uniformly distributed between -π/2 rad and π/2 rad, while the optic axis in the glioma region is mainly concentrated near 0 rad. By calculating the standard deviation of the optic axis value within the spatial window, the evaluation parameters of optical axis standard deviation are established. The range and mean value of optic axis standard deviation in normal brain tissue far exceed those in glioma tissue. The quantitative analysis underscores substantial distinctions of polarization information between tumor and normal mouse brain tissues.Within brain tissue, myelinated nerve fibers exhibit pronounced scattering and birefringence properties. The results of this study indicate that normal mouse brain tissue, rich in myelinated nerve fibers, is effectively identified by PS-OCT. The high-resolution intensity map contains detailed information, and the phase retardation highlights the birefringence changes of structures such as internal capsules and nerve fiber bundles. The optic axis values reflect the oriented arrangement of fibers, collagen, and other structures within the tissue. In the regions infiltrated by gliomas, the original structure of brain tissue is destroyed, the invading cancer cells decompose and reduce the expression of myelin, and the birefringence changes are weak. Compared with intensity images, polarization images exhibit higher contrast, providing a direct display of the distinctions between gliomas and normal tissue, making it easier to diagnose in clinical settings.Our preliminary study only used a limited number of mice. More samples and glioma tissues at different stages will be further studied in the future study. In addition, efforts will be made to investigate in vivo animal samples and ex vivo human specimens, aiming to promote the application of PS-OCT in the identification and resection of glioma in neurosurgery of the brain.ConclusionsOur results demonstrated that en-face images reveal structural information in the mouse brain, including the cerebral cortex, internal capsule, and hippocampus. Using polarization parameters of phase retardation and optic axis, the position and orientation of internal structures like the internal capsule and nerve fiber bundles with birefringent tissues can be clearly observed. The originally symmetric structure in the brain of tumor mice is destroyed, with erosion of the cortical edge and only partial visibility of internal fiber structures. Optic axis standard deviation proves effective in distinguishing between normal and glioma mouse brains. This study proves that high-resolution PS-OCT is promising to monitor the infiltration process of glioma through changes in birefringent tissues such as nerve fiber bundles.
ObjectiveSkin cancer is among the most common cancers worldwide. Skin cancer screening relies primarily on visual inspection by dermatologists, and largely depends on their experience. However, imbalances in regional development have led to an uneven distribution of medical resources worldwide. Telemedicine is an effective approach to alleviate this dilemma, and a major branch of this field is teledermatology. Dermatologists can remotely view clinical images and medical histories of skin cancer patients in various ways and provide remote diagnosis and treatment suggestions. However, teledermatology relies on medical experts and network conditions, and patients who cannot access the Internet in remote areas cannot enjoy the convenience of remote consultation. Some portable devices based on automatic diagnostic algorithms have made up for some shortcomings of traditional teledermatology; however, because they can only judge the type of disease, these devices have limited clinical applicability. To compensate for the shortcomings of the current research, our team design and build a skin tumor artificial intelligence-enhanced teleconsultation system, which has both offline automatic skin tumor screening and online remote consultation and preoperative planning functions.MethodsIn this study, we build a skin tumor intelligent teleconsultation system with two typical application scenarios: 1) skin tumor self-screening without network conditions. Patients or doctors who lack experience at the local site can use the dermatoscope in the system to obtain images of skin lesions and then use the deep learning algorithm in the system to generate automatic diagnosis results. 2) Remote skin tumor consultation under network conditions. Remote consultation for skin tumors involves real-time interactions among inexperienced doctors at a local site and experienced medical experts at a remote site. First, the doctor at the local site uses a dermatoscope or ordinary camera in the system to obtain skin images of patients with skin tumors. Then, the deep learning algorithm in the system generates automatic diagnosis results based on the images. Finally, the remote expert confirms the disease diagnosis result and recommends a corresponding treatment plan based on network-transmitted images and algorithmic cues. For patients requiring surgery, both parties can use the system for preoperative planning. Instructive annotations drawn by remote doctors on the screen are transmitted over the network to the local site and projected onto the body surface of the patient in situ. To achieve the above functions, we first train a RegNetY-800M model based on 7-point dataset and deploy it on Raspberry Pi, also using a neural network computing accelerator to accelerate neural network computation, then use camera, laser projector, and beam splitter to form a co-axial projective imaging design that can project instructive annotations made by remote experts with high accuracy onto patient's body surface, finally design a visual software interface for easy use by doctors. To characterize the system, we first design a benchtop experiment to quantify the achievable accuracy of the system, then design a control experiment to verify whether the system can improve the applicability and efficiency of the traditional teleconsultation system. Finally, a clinical experiment is designed to verify the clinical applicability of the system.Results and DiscussionsThe benchtop experimental results show that the maximum projection error of the system is less than 1.5 mm (Fig.4), and the CIEDE2000 value after color correction is less than 2, which can accurately restore colors in the scene (Fig.5). The control experimental results show that there is no significant difference between the algorithm in the system and dermatologists; dermatologists perform better with AI prompts. Clinical experimental results show that using this system for automatic diagnosis and remote consultation of skin tumors is feasible.ConclusionsThe intelligent teleconsultation system for skin tumors designed and built by our team has both automatic disease screening without a network and remote consultation with the network. The control experimental results show that the diagnostic results of the algorithm deployed in the system are comparable to those of dermatologists and that the algorithm can help dermatologists make more efficient and accurate decisions. The experimental results show that the system has clinical applicability. Compared to traditional remote consultation systems, this system has the following three advantages. 1) It has a wider application range. In remote areas without a network, the system can serve as a supplement to dermatologists, compensating for defects in which traditional teleconsultation systems cannot be used without a network. 2) Better performance. Automatic diagnosis results in the system can assist specialists in a more efficient and accurate screening of skin tumors. 3) Intuitive display. This system can project annotations made by remote experts onto body surfaces of patients with high precision, thereby making remote guidance in preoperative consultations and surgical planning processes more intuitive and precise. This system can help patients in areas with scarce medical resources perform early screening for various diseases, such as skin tumors.