Abstract
Blood vessel imaging provides crucial information for physiological monitoring and disease diagnosis. Optical-resolution photoacoustic microscopy (PAM) is gaining intense attention in high-resolution in vivo imaging of blood vessels. However, its applicability in clinical settings is hindered by the requirement of bulky and costly pulsed lasers as well as complex alignment procedures. Here, as a novel approach to high-quality and cost-effective microvasculature imaging, a dual-wavelength fiber-coupled laser diode-based PAM (FC-LD-PAM) system is demonstrated on the mouse ear and brain in vivo. Furthermore, assisted with a deep learning method, the proposed technique effectively combines the advantages when using both small and large core-size multimode fibers—successfully enhances the resolution of blurry mouse microvascular images obtained using a large core-size fiber, revealing fine details that are comparable to those achieved using a small core-size fiber; significantly reduces the overall time for data acquisition up to fourfold, improving the efficiency significantly in the imaging process. In addition, the proposed approach can achieve accurate and high-resolution mapping of oxygen saturation in vivo, providing functional insight on living tissue. Therefore, the FC-LD-PAM can serve as a translational tool for high-resolution imaging with enhanced accessibility and versatility in the biomedical field.
1 Introduction
Blood vessels play an important role in circulatory networks, transporting, and supplying oxygen and nutrients, which carry vital information for physiological monitoring and disease diagnosing. Therefore, blood vessel imaging enables medical professionals to screen, diagnose, and prognose tumor growth and metastasis.[1, 2] Various imaging modalities have been developed to visualize the vasculature and microvasculature. For example, non-optical methods, including X-ray/computed tomography,[3] magnetic resonance imaging,[4] and ultrasound imaging,[5] are mature tools to examine blood vessels. However, these techniques are commonly utilized for large blood vessel imaging and are not effective for microvasculature imaging due to their poor spatial resolution and contrast. Whereas, optical methods, including confocal fluorescence microscopy,[6] two-photon fluorescence microscopy,[7] optical coherence tomography,[8-10] and laser speckle contrast imaging,[11] are widely used for microvasculature imaging due to their high spatial resolution. However, most of them require fluorescent labeling to improve the contrast. To achieve label-free and in vivo microvascular imaging, optical-resolution photoacoustic microscopy (OR-PAM), as a hybrid method combining high optical absorption contrast with ultrasound detection, has been developed, which attracts significant interest.
OR-PAM is a label-free and noninvasive imaging technique based on photoacoustic (PA) effect. In blood vessel imaging using OR-PAM, two representative endogenous absorbers, oxy- and deoxy-hemoglobin (HbO2 and HbR, respectively), absorb the incident light energy and induce temperature rise, in turn generating PA waves (also known as PA signals) through thermoelastic expansion. By detecting the PA signals, OR-PAM enables anatomical and functional imaging (e.g., oxygen saturation of hemoglobin (sO2), flow speed, and metabolic rate of oxygen), which has been gaining widespread attention in recent years.[12-20] Typically, pulsed lasers, such as Q-switched diode-pumped solid-state lasers, Ti:sapphire lasers, and optical parametric oscillators, are used in OR-PAM systems, generating high-energy light pulses with a short pulse width to obtain PA signals with high signal-to-noise ratio (SNR). However, the high cost, bulky size, and high-level laser-safe requirement of these lasers prevent their wide usage in clinical environments. Recently, laser diodes (LDs) have become promising alternative laser sources for PAM systems due to their economical price and compact size, promoting the development of PAM into more cost-effective and miniaturized forms.[21-28] For example, a low-cost 905-nm pulsed LD-based PAM (LD-PAM) was developed for biological tissue imaging.[21] Blood tubes and mouse ears were imaged with a high resolution of ≈7 µm, demonstrating the great potential of LD-PAM for clinical applications. However, 128 signal averaging was required to achieve ≈13 dB of SNR, and more averages were needed to further improve the SNR. Another LD-PAM system with an optical scheme was developed to improve the imaging SNR, which combined aspheric and cylindrical lenses to efficiently collimate and focus the excitation light beam of a 905-nm pulsed LD.[22] The vasculature on ex vivo mouse ear and porcine ovarian tissue were imaged. However, it is difficult to visualize microvasculature due to the limited resolution, showing some discontinuous or mixed blood vessels in PAM images. Most near-infrared (NIR) pulsed LD-PAM systems suffered from relatively low image quality. This is primarily due to the fact that the absorption coefficient of hemoglobin is lower in the NIR range compared to the visible range. Additionally, the inherently low power output of most NIR pulsed LDs further contributes to the challenge of obtaining high-quality PA images. Besides, continuous-wave LDs in pulsed mode have also been investigated in PAM due to the increased light energy and broad wavelength range of the continuous-wave LDs, especially for microvasculature imaging.[26, 27, 29] However, many existing LD-PAM systems have simply employed high-numerical aperture (NA) lenses to focus the laser beam to achieve high-resolution imaging. This approach may not be the most optimal, as the emitter sizes and inherent light beam characteristics of LDs need to be carefully considered and accounted for in the optical system design. To address this issue, our previous work optimized the optical system design by reshaping the LD beams to develop a high-speed and high-resolution LD-PAM system for in vivo microvasculature imaging, achieving a high lateral resolution of 4.8 µm with a 30 kHz A-line rate.[26] With a proper overdrive circuit implementation for LDs, the system can obtain high SNR PAM images without signal averaging. In addition to anatomical imaging, we further optimized the LD-PAM system in a reflection mode by a ring-shaped ultrasonic transducer and achieved functional imaging (e.g., sO2) using two LDs with different wavelengths.[27] High-quality microvasculature imaging and accurate sO2 calculation can be achieved with this low-cost PAM system, which facilitates the adoption of PAM in both preclinical and clinical applications. However, the alignment of the set of lenses to reshape the laser beam in free space is not trivial. In this regard, fiber optic technologies are employed to improve the compactness of the PAM systems.[30-33] The majority of these systems are laser-based PAM systems. Recently, a new OR-PAM system was developed with the pulsed LD beam homogenized and shaped by a square-core multimode optical fiber.[34] However, only ex vivo experiments were demonstrated in this work, which can only visualize the blood vessels with a limited lateral resolution. Therefore, the performance and applications of the system are limited.
To address the challenges, here, we demonstrate a reflection-mode fiber-coupled dual-wavelength LD-PAM (FC-LD-PAM) system for in vivo microvascular imaging and sO2 measurement. Non-perfect point sources generated by the two LDs are coupled to a multimode fiber and reshaped into sources with higher quality, achieving a high lateral resolution of ≈7 µm. The use of multimode fibers makes the system alignment simple, improving the compactness and flexibility of the system. In vivo microvasculature imaging and sO2 mapping of mouse ear and brain were conducted to demonstrate the feasibility of the proposed low-cost and compact FC-LD-PAM system. Furthermore, the performance of FC-LD-PAM varies depending on the core size of the multimode fibers used. A small core-size multimode fiber facilitates high-resolution imaging, whereas a large core-size multimode fiber enables higher coupling efficiency, leading to increased power output and higher SNR. This, in turn, enables high-quality and high-speed imaging. There is a demand for a method that can effectively combine the advantages offered by both small and large core-size multimode fibers. Additionally, recent advancements in deep learning applications have further improved the overall performance of traditional laser-based PAM systems, including contrast enhancement,[35] super-resolution,[33, 36, 37] style transformation,[38] and increasing penetration depth.[33] Therefore, we propose to apply a transformer-based deep learning method to transform low-resolution PA images with high contrast, obtained using the large fiber, to high-resolution ones, which are comparable with the small fiber results while maintaining the high contrast. The deep learning-assisted FC-LD-PAM system holds promising prospects in the development of compact, cost-effective, and robust devices for clinical imaging translation and applications.
2 Material and Methods
2.1 FC-LD-PAM System
In OR-PAM imaging, the lateral resolution is highly determined by the focused light beam quality on the samples. In consideration of the fact that the emitter size of the non-ideal point source generated by the LDs is 15 µm × 1 µm, two cylindrical lenses and an iris are used to reshape the light of each LD to be a circular and collimated beam successfully in our previous study.[27] To simplify and optimize the system, here, we develop an FC-LD-PAM system (Figure 1a). The entire system consists of five parts: 1) the two visible LDs for excitation, 2) multimode fiber combined with optical components for beam reshaping and light delivery, 3) linear motorized stages for scanning, 4) the water tank and sample holder for sample placement, and 5) a ring-shaped ultrasonic transducer with an amplifier and a computer for data acquisition, processing, and display. The system uses two continuous-wave LDs (NDB7675 and NDG7575, Opt Laser Inc.), with central wavelengths of 460 and 517 nm (Figure S1, Supporting Information), enabling label-free microvasculature imaging and sO2 measurement via a spectral unmixing algorithm.[39, 40] Pulse drivers (LMG1020EVM, Texas Instruments Inc.) pulsed drive each LD with a repetition rate of 10 kHz, a pulse width of ≈10 ns, and a time delay interval between two LDs of 800 ns. After collimation using aspheric lenses (A240TM-A, Thorlabs, Inc.), two elliptical beams are obtained due to the different emitter sizes in vertical and horizontal directions, and then they are combined by a dichroic mirror (DMSP490, Thorlabs Inc.) and coupled into a multimode fiber (M64L01 or M42L01, Thorlabs Inc.) by a fiber coupler (F240FC-532, Thorlabs Inc.). The connected multimode fiber is used to reshape the combined beam to a circular beam and transmit the light to the target, making the system flexible and compact. The input and output beam shapes of the multimode fiber are shown in Figure S2 (Supporting Information). Then, the output beam from the multimode fiber is collimated by an achromatic doublet (AC127-025-A-ML, Thorlabs Inc.) and focused by an objective lens (LMU-5X-NUV, Thorlabs Inc.) onto the sample to induce PA waves. The optical simulation and chromatic focal shift analysis are shown in Figure S3 (Supporting Information). A ring-shaped ultrasonic transducer (Focal length: 6.3 mm; center frequency: 40 MHz; −6 dB bandwidth: 89%), between the objective lens and the sample, is confocally and coaxially aligned with the optical path to detect the excited PA signals from the sample, acting as an FC-LD-PAM probe. The ring-shaped ultrasonic transducer is immersed in a customized water tank which is covered by a thin membrane at the bottom as a transparent window. The sample is coupled to the membrane using ultrasound gel, allowing PA signals to propagate from the sample to the ultrasonic transducer. The detected PA signals are then amplified by low noise amplifiers (ZFL-500LN-BNC+, Mini-Circuits), filtered by a low pass filter (BLP-70+, Mini-Circuits), and delivered to a computer for data acquisition and processing. For wide-field imaging, the FC-LD-PAM probe is fixed to a 2-axis linear motorized stage (L-509.10SD00, Physik Instrumente (PI) Singapore LLP) for raster scanning. Note that the scanning movement disturbs the surface of the water which will scatter the light before it focuses on the sample. As shown in Figure 1b, we added a thin glass between the objective lens and ultrasonic transducer to ensure a flat water surface so that the light passes through and then can be well focused on the sample. The data acquisition program combined with laser and motor trigger control is developed in LabVIEW software (LabVIEW 2017, National Instruments, USA).
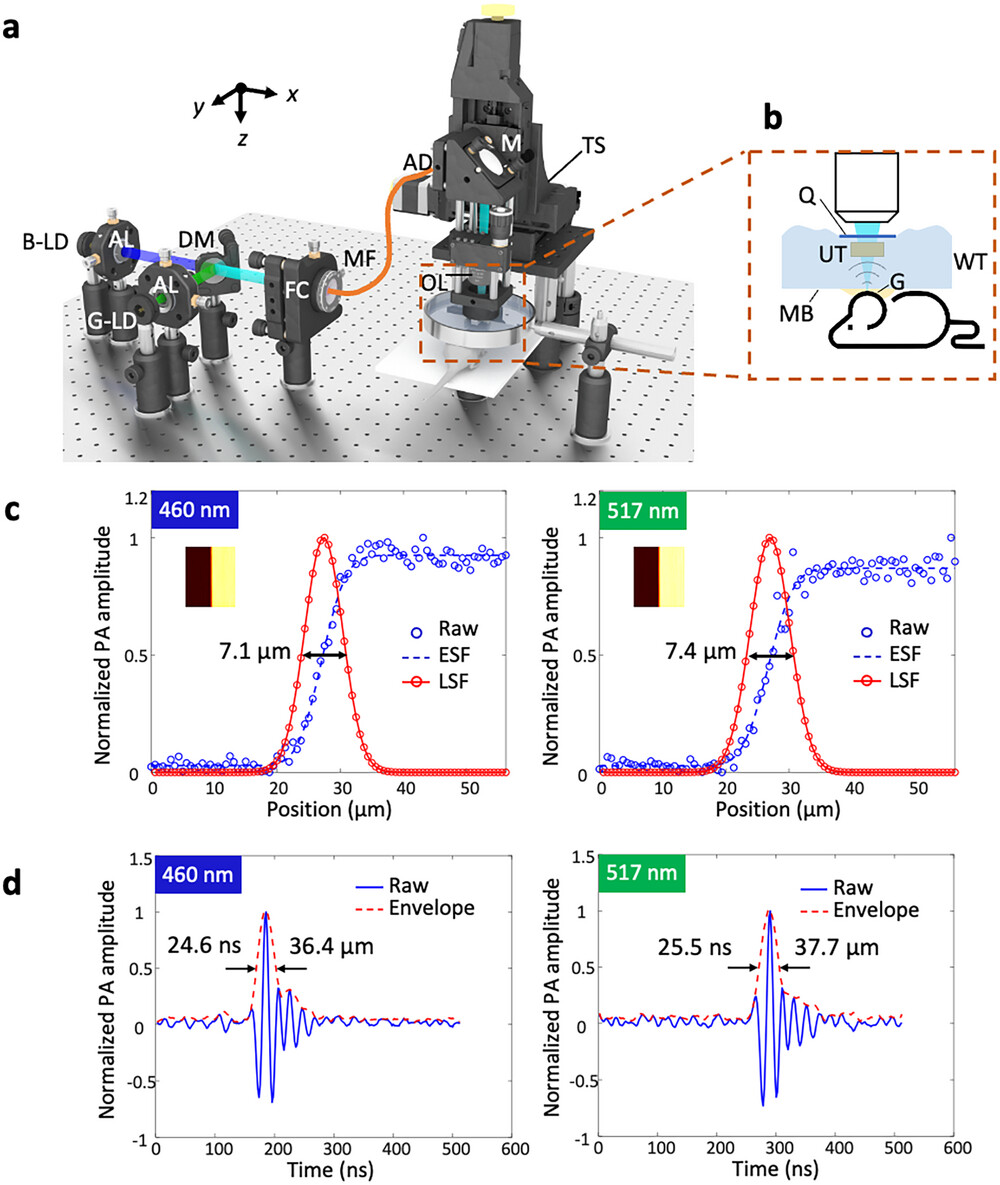
To evaluate the performance of the FC-LD-PAM, we quantitatively measured the lateral and axial resolutions. We imaged a sharp edge to obtain a PA maximum amplitude projection (MAP) image (Figure 1c). We applied an error function to fit the measured data, resulting in the derivation of an edge spread function (ESF). Subsequently, we calculated the first-order derivative of the ESF to acquire the line spread function (LSF). The lateral resolution is defined by the full width at half maximum (FWHM) of the LSF from the cross-sectional profile line of the PA MAP image. From Figure 1c, the lateral resolutions of the blue and green LD beams are estimated to be 7.1 and 7.4 µm, respectively, which are close to the theoretical value (≈7.9 µm). The axial resolutions were evaluated from the enveloped A-line signals, resulting in FWHM of ≈36.4 and ≈37.7 µm for blue and green LD beams, respectively.
2.2 Preparation of Animals
For in vivo mouse ear imaging, mice were placed on the flat holder and anesthetized with ≈1.5% isoflurane mixed with oxygen at a flow rate of 0.6 L min−1 during the experiments. The mouse ear was clinging to the membrane window of the water tank using ultrasound gel. For in vivo mouse brain imaging, the mice were positioned and anesthetized similarly. To expose the targeted brain for imaging, we made a 5 mm × 5 mm cranial window on the skull.
All animal experiments were conducted in conformity with laboratory animal protocols approved by the Health, Safety, and Environment Office of the Hong Kong University of Science and Technology (HKUST) (license number: AEP20220010, AEP20220084).
2.3 Resolution Enhancement via Deep Learning
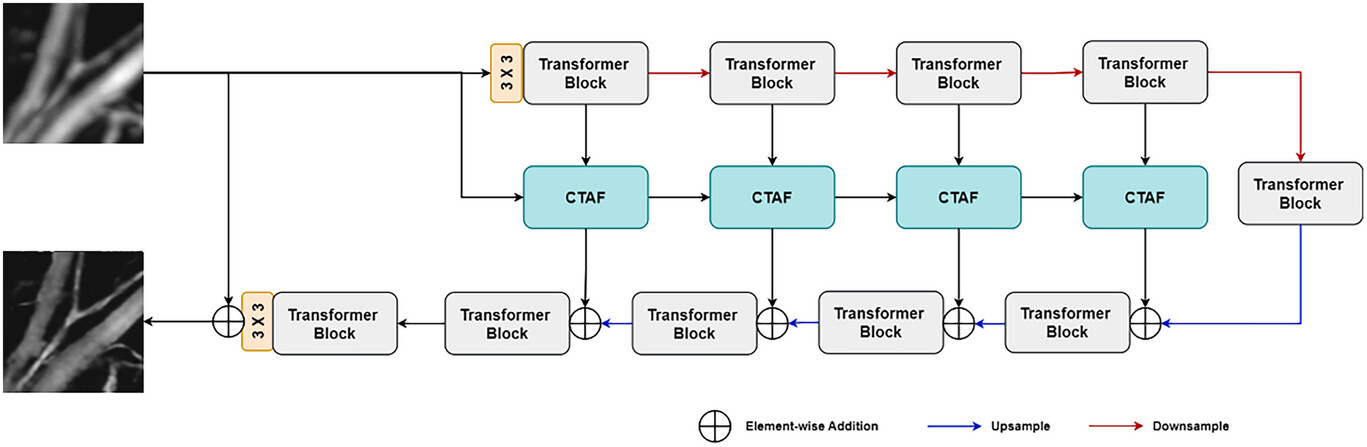
The downstream process of encoding often involves downsampling of the image, potentially leading to the loss of high-frequency details. To mitigate this issue, we propose the CTAF block, as shown in Figure S4b (Supporting Information). This block is designed to weigh the output of skip connections using the previous high spatial dimension encoder output, thereby helping to preserve fine-grained details. The block employs a multi-depth-wise convolution attention mechanism.[45] The feature map
The proposed architecture is end-to-end trainable in PyTorch and executed on two NVIDIA GeForce RTX 3090 GPUs. It operates in an end-to-end learning fashion, eliminating the necessity for costly large-scale pretraining. In the experiment, we employed a 4-level encoder-decoder. From level 1 to level 4, the number of transformer blocks is 1,2,8,8, and the refinement stage contains 4 blocks. We trained our models with AdamW[46] optimizer with the momentum terms of 0.9 and 0.999 for 500 epochs with cosine decay[47] strategy that decreases the learning rate at 1e-6 with initial learning rate 2e-4. The training data contains 1100 pairwise images cropped from a set of large-scale mouse ear and brain blood vessel images. We started training with a patch size of 256 × 256 and a batch size of 4. For data augmentation, we used horizontal and random rotations. For testing, a dataset consisting of ten mixed mouse ear and brain images was prepared, showing the versatility of the trained model in accommodating different tissues.
3 Results
3.1 In Vivo Mouse Ear and Brain Imaging
To evaluate the performance of the proposed low-cost and flexible FC-LD-PAM system for in vivo high-resolution microvasculature imaging and sO2 measurement, mouse ears, and brains were imaged. The scanning step size along the x-axis and the y-axis are both 2.5 µm.
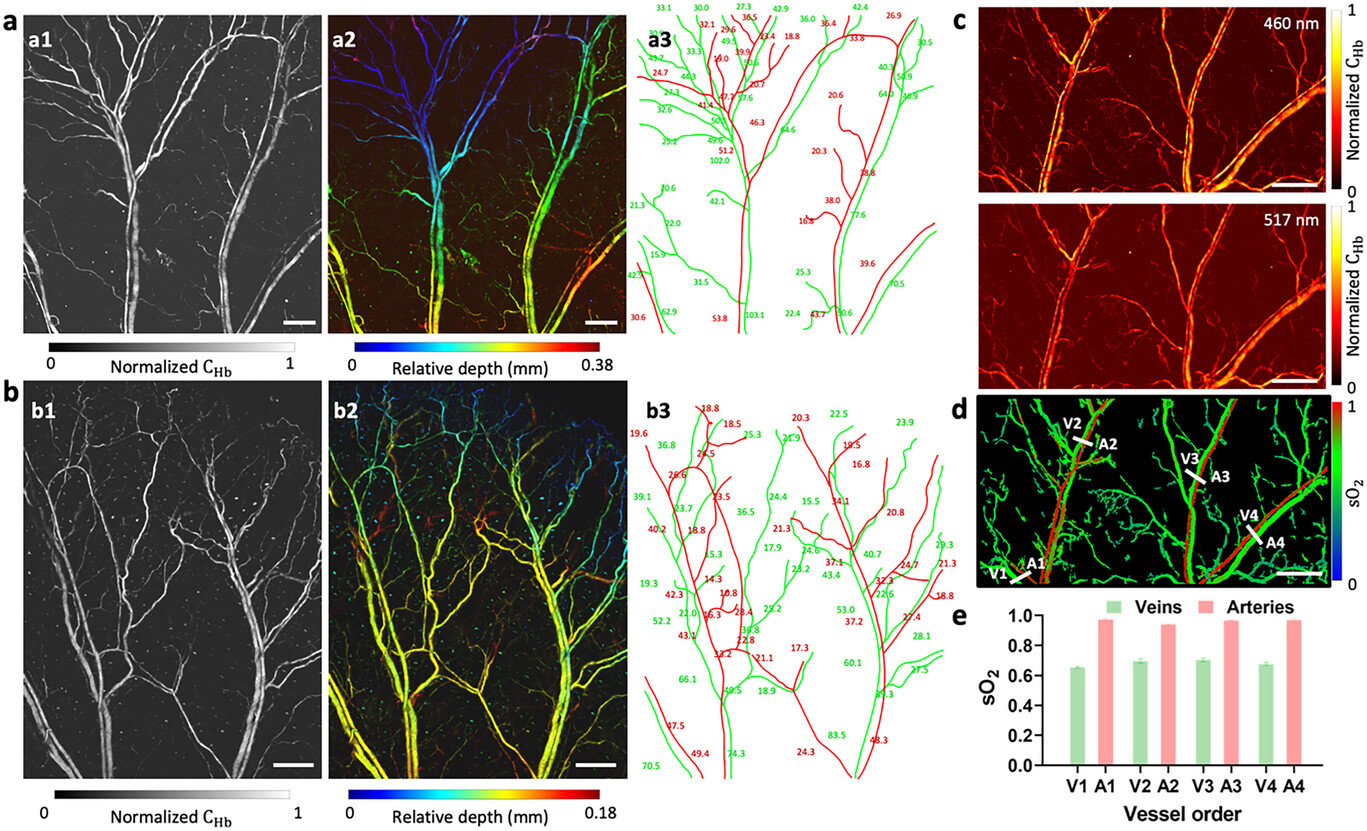
The in vivo PA images of two mouse ears acquired with the FC-LD-PAM system are presented in Figure 3a,b, with different region sizes of 5 mm × 5 mm (Figure 3a) and 3.8 mm × 5 mm (Figure 3b). MAP images are shown in Figure 3a1,b1, where the structure of microvasculature networks can be clearly observed. Figure 3a2,b2 depicts pseudo-color-encoded vascular depth, demonstrating the ability of the system to manifest 3D variations in microvasculature. Vessel segmentation reveals dynamic changes in vessel diameter along with bifurcations in the arterial and venous trees (Figure 3a3,b3). The observed non-uniform intensity distribution in the blood vessel images shown in Figure 3b1,b2 reflects the inherent characteristics of the imaged vascular anatomy, such as vessel diameter. As shown in Figure 3b3, the vessel diameter decreases from the lower half to the upper half of the images, and the upper half also contains a greater density of smaller capillaries. Therefore, upper half regions with a lower concentration of hemoglobin will exhibit weaker optical absorption and, consequently, appear darker in the images. In addition to vascular anatomical information, the dual-wavelength FC-LD-PAM system can also be used to obtain functional information. Figure 3c shows FC-LD-PAM MAP images of a mouse ear under blue and green LD excitations. The sO2 values were calculated from the dual-wavelength high-resolution images using a linear-mode-based spectral fitting method (see Note S1, Supporting Information) on each pixel. Morphological operations and transforms were employed on the 460 nm PAM image to eliminate the background noise. These processes resulted in a binary mask that was then applied to the calculated sO2 image, yielding a sO2 image (Figure 3d) that is free from background interference.[48] We quantified the sO2 values in four vein-artery pairs as labeled in white lines in Figure 3d. Figure 3e shows the sO2 values of each vein and artery, which agrees with the typical sO2 values of veins (0.65−0.75) and arteries (>0.95).[49]
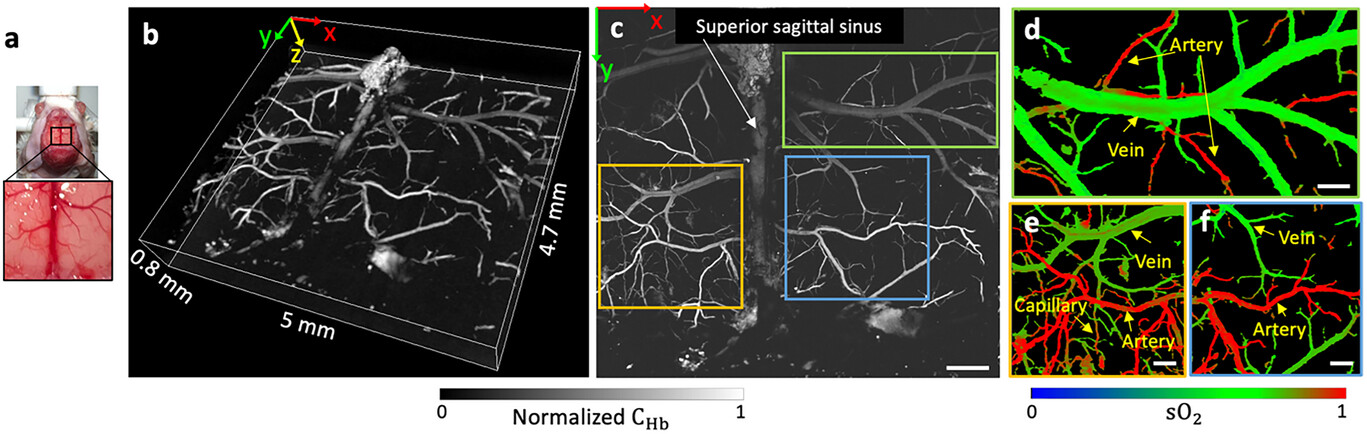
For mouse brain imaging, the mouse was positioned and anesthetized similarly. A ≈5 mm × 5 mm cranial window was imaged with parietal craniotomy (Figure 4a). The structural information can be clearly viewed in both direct 3D rendering (Figure 4b) and MAP image (Figure 4c). Figure 4b shows the FC-LD-PAM's capacity in depth-resolved 3D imaging of microvasculature networks, which takes ≈12.5 min to acquire this volumetric image. Superior sagittal sinus, which is an unpaired venous structure along the attached margin of the falx cerebri, can be identified (Figure 4c, white arrow) as well as other microvascular structures. Figure 4d–f displays the sO2 images of three magnified regions from Figure 4c, where arteries, veins, and capillaries can be identified based on the respective sO2 values and vessel diameters. Therefore, our technique provides comprehensive visualization of brain vasculature and enables quantification of sO2 levels in cerebral blood vessels, allowing further investigations into the intricate relationship between vascular dynamics and neuronal activity.
Assuming the optical focus is 200 µm beneath the skin surface, the optical fluence estimated at the tissue surface for all in vivo experiments are ≈2.8 and ≈1.0 mJ cm−2, respectively, for two LDs with pulse energies of ≈29 and ≈20 nJ, which maintained lower than the American National Standard Institute safety limit of 20 mJ cm−2.[50]
3.2 Deep Learning-Enhanced In Vivo Mouse Ear and Brain Imaging
The experiments above were performed using a multimode fiber with a small core size of 10 µm, which can achieve a high resolution of ≈7 µm (Figure 1c) to visualize arteries, veins, and most capillaries.[51] However, the small core-size fiber's low coupling efficiency (≈25%) led to the blockage of a substantial portion of the light energy. Consequently, the resulting PA images suffered from a diminished contrast-to-noise ratio (CNR). To address the issue, we replaced the small core-size fiber with a different multimode fiber featuring a large core size of 50 µm with a coupling efficiency of ≈85%, resulting in illumination energies of ≈90 and ≈70 nJ for green and blue LDs, respectively. However, this change resulted in a trade-off as the resolution was sacrificed, reducing it to ≈30 µm (Figure S5, Supporting Information). This is because the larger core diameter of the 50-µm fiber results in a larger focused spot size, ultimately limiting the achievable lateral resolution compared to the tighter focusing enabled by the smaller 10-µm fiber core (see Note S2, Supporting Information).
To consider the benefits of both small and large core-size fibers, we applied a deep learning method (see Material and Method, Figure 2; Figure S4, Supporting Information) to transform low-resolution PA images with high CNR, obtained using the large core-size fiber, to high-resolution ones, which are comparable with the small core-size fiber results while maintaining high CNR. Besides, when using large core-size fibers, a larger scanning step size can be implemented during the scanning process compared to the small core-size fiber, which increases the imaging speed and reduces storage costs for a given region. For example, it takes ≈12.5 min to cover a 5 mm × 5 mm region when using the small core-size fiber for high-resolution imaging. However, the same region can be imaged in 3 min only when using the large core-size fiber with a larger scanning step size, which significantly reduces the overall imaging time, while preserving the high image quality for both resolution and contrast.
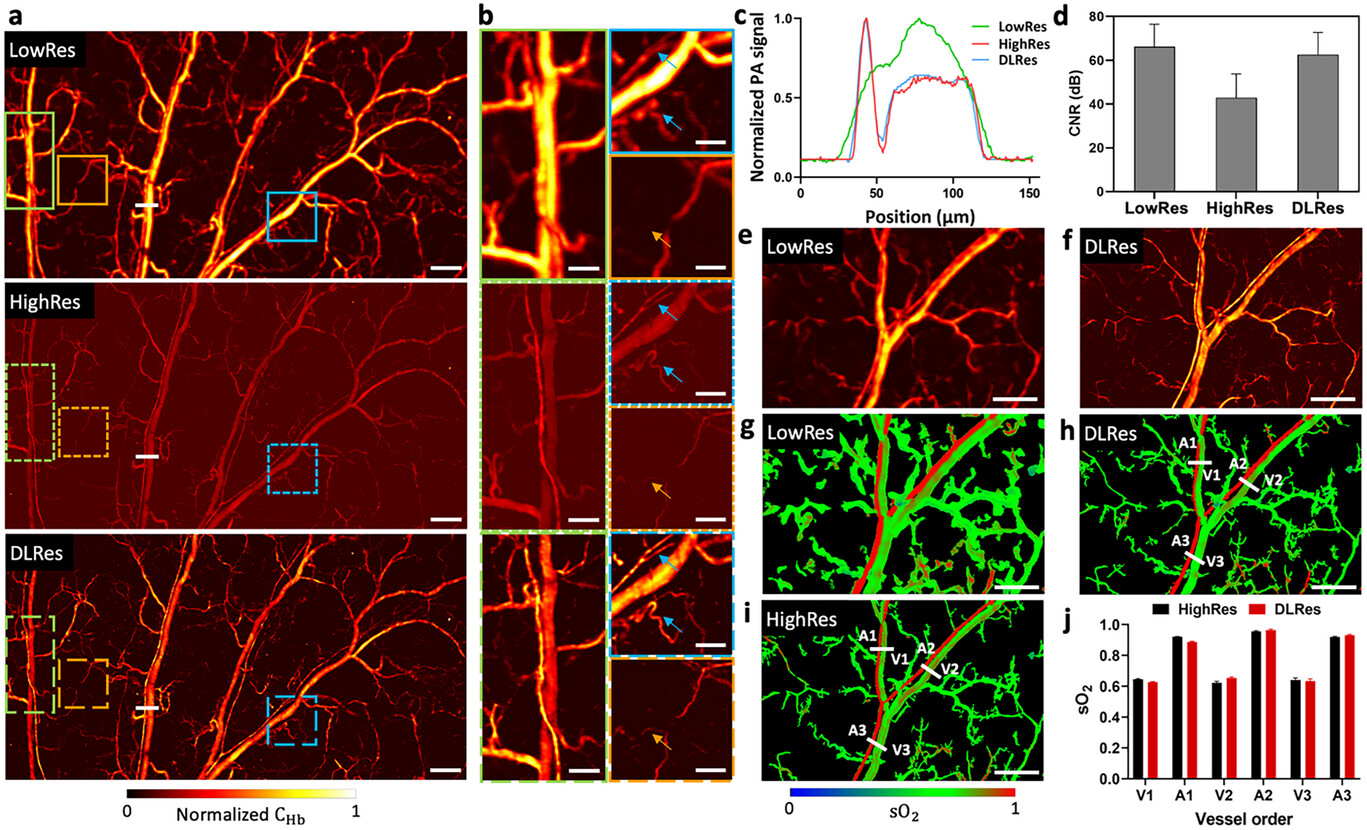
Figure 5a shows a FC-LD-PAM low-resolution (LowRes) image with a core size of 50 µm, high-resolution (HighRes) image with a core size of 10 µm, and deep learning-based high-resolution (DLRes) image of a mouse ear with a region of 7.5 mm × 4 mm. We can observe that the LowRes image exhibits a high CNR, indicating a clear distinction between the signal and background noise. However, its low resolution results in reduced fine details and spatial accuracy. On the contrary, the HighRes image showcases a higher resolution with a lower CNR. To improve the resolution and maintain the high CNR of the LowRes image, the DLRes image from the LowRes image was obtained by a deep learning method. The DLRes image combines the advantages of high resolution and high CNR, resulting in an appealing image that exhibits both fine details and a clear distinction between the signal and background noise. Three zoomed-in regions (Figure 5b) specifically show the feasibility and reliability of the LowRes-to-HighRes transformation process by our deep learning algorithm. For instance, for the green regions in Figure 5b, the LowRes image fails to reveal the closely spaced parallel and crossed blood vessels, which can be seen in the DLRes image, showing a structural consistency with the HighRes ground truth image. In the blue regions, some blurred vascular structures (denoted by blue arrows) in the LowRes image have been resolved in the DLRes image. In the orange regions, the orange arrow indicates a single capillary which is barely seen in the LowRes image, appearing as a faint shadow, but is discernible in the DLRes image, matching the capillary's appearance in the HighRes ground truth image. In addition, as shown in Figure 5c, we compared the cross-sectional profiles of representative vessels in the B-scans indicated by the white solid lines in Figure 5a. The plot is relatively smooth over the area that contains a vein-artery pair due to the limited resolution in the LowRes image, while the plot of DLRes indicates the capability of distinguishing closely packed vessels, aligning with the HighRes ground truth image. To quantify the resolution improvement achieved with the deep learning approach, we compare the FWHM of the typical capillary features in the LowRes, HighRes, and DLRes images (Figure S6, Supporting Information). An average of 4.4 times resolution improvement was demonstrated by DLRes images over LowRes images. To further characterize the outstanding performance of the DLRes images, the metric CNR among these images is provided for comparison (Figure 5d). The mean CNR values for the LowRes, HighRes, and DLRes images are 66.2, 42.9, and 62.6 dB, respectively. There is a slight deterioration in CNR for DLRes images compared with LowRes images. The process of recovering high-frequency details can amplify noise and introduce artifacts, which can potentially have a negative impact on the final CNR. However, in our proposed method, efforts have been made to maximize the preservation of high CNR while simultaneously improving the resolution. Therefore, the DLRes images can unveil comparable fine details to the HighRes ground truth images, exhibiting ≈19.7 dB improvement in CNR. Figure 5e,f shows LowRes and DLRes PAM images, respectively, of another mouse ear microvasculature with corresponding sO2 maps (Figure 5g,h). The sO2 map of the DLRes image was obtained by applying a mask obtained from the DLRes image onto the sO2 map of the LowRes image. The DLRes sO2 map showcases a high consistency with the HighRes sO2 map (Figure 5i). Then, we chose three vein-artery pairs in both the DLRes sO2 map and the HighRes sO2 map to calculate the average sO2 values for comparison, as shown in Figure 5j, indicating that the proposed method is capable of providing a comparable sO2 map to the HighRes sO2 map perceptually and quantitatively. In certain instances, as shown in Figure S7 (Supporting Information), there are some sO2 calculation errors in the HighRes images. The main reason for the miscalculation of sO2 in the images acquired using the 10-µm fiber is the inherently lower CNR of these images compared to those acquired using the 50-µm fiber. The lower CNR in the 10-µm fiber images makes it more challenging to accurately resolve the subtle differences in optical absorption between HbO2 and HbR, which leads to errors in the calculated sO2 values. In contrast, the LowRes and DLRes sO2 maps, which are both derived from the 50-µm fiber data, would provide more reliable sO2 quantification.
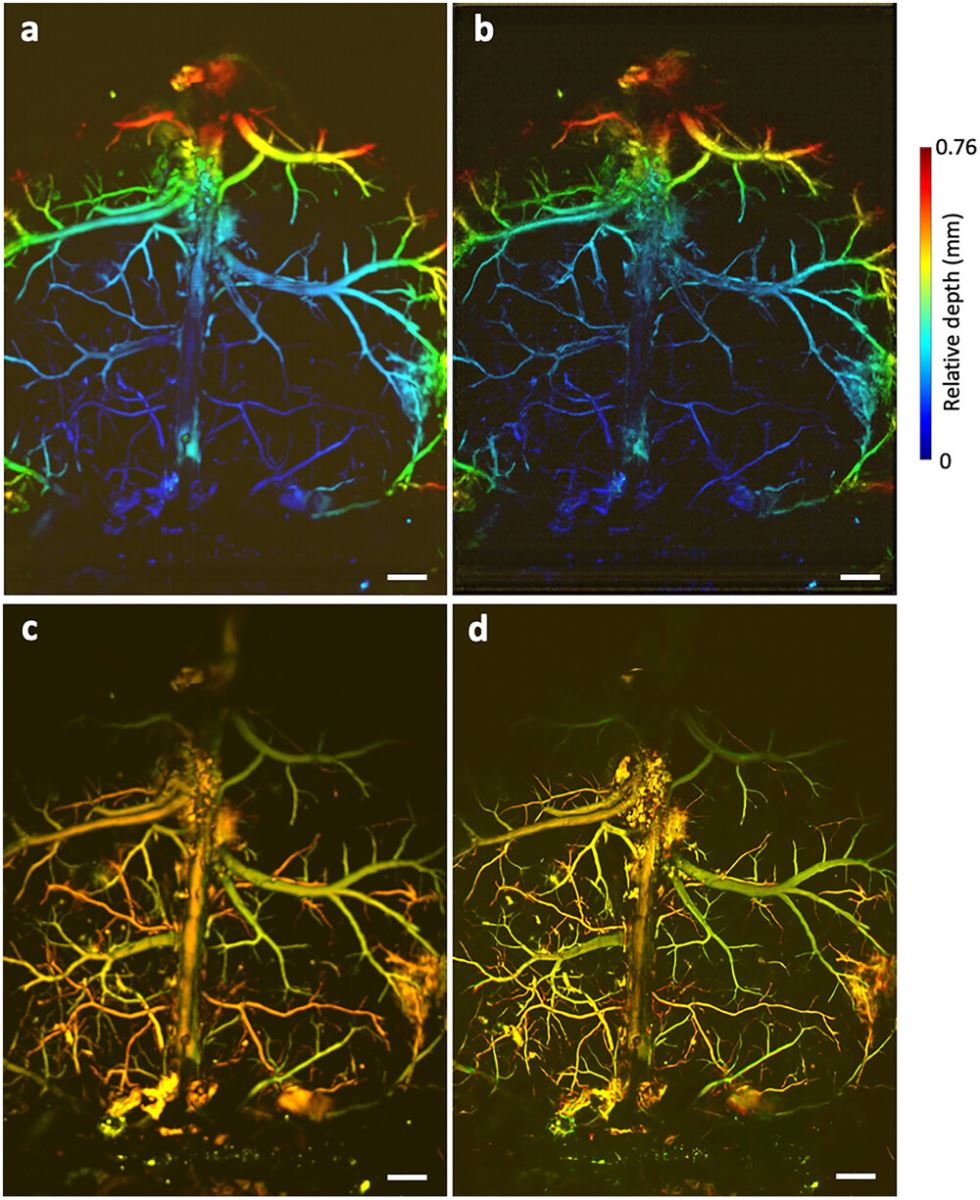
The proposed approach was also validated on in vivo mouse brain imaging. Figure 6 and Figure S8 (Supporting Information) show the original LowRes and DLRes depth-resolved images (Figure 6a,b), dual-wavelength overlapping MAP images (Figure 6c,d), as well as the sO2 maps of mouse brain vasculature (Figure S8i–n, Supporting Information). The DLRes images exhibit improved detail, clarity, and fidelity to the original anatomical structures. Moreover, the method successfully maintains a high CNR, preserving the visibility of important brain features while minimizing the influence of noise and artifacts. These enhanced results hold immense potential for advancing neuroscience research, enabling more precise analysis, and facilitating detailed qualitative and quantitative assessments of mouse brain structures and functions.
4 Discussion
Conventional pulsed laser-based PAM systems demonstrate significant advantages, such as high CNR and high resolution. However, our FC-LD-PAM system, while exhibiting a lower lateral resolution of ≈7 µm compared to the ≈3 µm resolution of most conventional laser-based systems, is still able to adequately resolve the majority of the blood vessels of the mouse ear and brain. With sufficient resolution and comparable CNR, our FC-LD-PAM has great potential for clinical applications, which enables high-quality in vivo microvasculature imaging and sO2 mapping using two cost-effective LDs. The blue and green LDs cost USD 137 and USD 267, respectively, accompanied by two pulsed drivers (USD 198 for each). This results in a total cost of USD 800 for the excitation source, which is significantly more affordable. Compared to conventional pulsed lasers that are commonly used in PAM systems, the cost reduction ranges from 25 to 50 times. Besides, the excitation source part in our system can be packed in a small size due to the compactness of the LDs (11 mm in length and 9 mm in diameter) and the drivers (49 mm × 40 mm × 22 mm). Multimode fibers are used in the excitation source, which greatly further improves the compactness and flexibility of our proposed FC-LD-PAM system. The multimode fiber with a core size of 10 µm is employed to achieve optimal image resolution for in vivo microvasculature imaging. On the other hand, using the multimode fiber with a core size of 50 µm offers a notable advantage in terms of light coupling efficiency. The coupling efficiency is three times higher compared to the 10 µm multimode fiber, resulting in improved CNR in the acquired PAM images. Furthermore, the use of the multimode fiber with a large core size allows easy optical alignment, further enhancing the overall performance and usability of the system. To combine both advantages, we propose a deep learning algorithm to transform the blurry (LowRes) images obtained with the large core-size fiber to match the details in the high-resolution (HighRes) images obtained with the small core-size fiber. Meanwhile, the resulting DLRes images maintain a high CNR. It is worth mentioning that deep learning approaches allow for a more tailored and robust solution compared to general denoising algorithms. Additionally, utilizing a large core-size imaging apparatus combined with deep learning enables the attainment of high-resolution and high-CNR imaging even with a larger scanning step size. This advantageous feature results in a significant reduction in the total imaging time, thereby enhancing overall imaging efficiency. This represents a practical advantage in terms of improved clinical applicability and faster data acquisition. Furthermore, the compact size and cost-effectiveness of our FC-LD-PAM system present unique opportunities for practical applications that may be challenging to achieve with traditional PAM approaches. For instance, the small footprint and low power consumption of the FC-LD-PAM could enable the further development of wearable PAM devices for continuous monitoring or ambulatory settings. Furthermore, the affordability of the FC-LD-PAM system makes it well-suited for deployment in resource-limited environments, such as remote clinics or field research settings in developing regions. By leveraging these distinctive characteristics, FC-LD-PAM has the potential to transform the accessibility and modalities of PAM imaging capabilities, ultimately enhancing patient care and expanding the frontiers of scientific discovery in a variety of contexts.
Although our proposed technique has shown promising abilities in high-resolution and high-CNR microvasculature imaging and sO2 mapping, it is imperative to acknowledge its existing limitations and further refinement which is necessary to fully exploit its capabilities. First, the LD repetition rate in the current system is 10 kHz, which can be up to 50 kHz theoretically. Therefore, the imaging speed can be further increased by using fast scanning methods (e.g., microelectromechanical system[52] and resonant-galvo scanner[53]) for high-speed and large field-of-view imaging. Second, it is important to note that the quality of deep learning output relies on the quality of the input data. For example, when dealing with input images that contain severely blurred capillaries, the network may fail to accurately restore all the intricate details. Consequently, our objective is not to achieve a flawless restoration of all details in the LowRes image, but rather to approximate the resolution of the HighRes ground truth image to the best possible extent. To further enhance the accuracy and effectiveness of the imaging transformation, acquiring additional PAM image data for training the network will be beneficial. Third, our current demonstration was conducted solely on normal mice. To establish the diagnostic reliability of our proposed technique, the disease model should be carried out in the following work, which will enable us to evaluate the performance and effectiveness of our technique in a clinical context. Finally, in addition to hemoglobin concentration and sO2, blood flow is also a crucial functional parameter for diagnosing and staging numerous diseases. To enable multifunctional imaging using our proposed technique, the measurement of blood flow speed can be considered for future research. This can be achieved by leveraging the Grueneisen relaxation effect.[54] Meanwhile, advanced segmentation methods and more sophisticated masking algorithms can be investigated to better isolate the vascular structures while minimizing the loss of spatial information, which can be used for better visualization of functional parameters (e.g., sO2 and blood flow).
5 Conclusion
In summary, we proposed a low-cost high-resolution fiber-coupled dual-wavelength LD-based PAM system assisted with deep learning that enables high-quality microvasculature imaging and sO2 mapping. The versatility of FC-LD-PAM is experimentally demonstrated, showing great potential as a low-cost imaging platform for physiological monitoring and disease diagnosis. In addition, the feasibility and reliability of the proposed deep learning algorithm to enhance the imaging resolution is also demonstrated on mouse ears and brains in vivo, providing new insights to expand the application of the proposed FC-LD-PAM system in biomedical fields.
Acknowledgements
The authors acknowledge the funding support of The Hong Kong University of Science and Technology startup grant (R9421).
Conflict of Interest
T.T.W.W. has a financial interest in PhoMedics Limited, which, however, did not support this work. The remaining authors declare no competing interests.
Author Contributions
B.H., X.L., and T.T.W.W. conceived of the study. B.H. and X.L. built the imaging system. B.H. and V.T.C.T. prepared the specimens involved in this study. B.H. performed imaging experiments. S.C.K.C. participated in algorithm optimization. B.H. processed and analyzed the data. B.H., S.C.K.C., and T.T.W.W. wrote the manuscript. T.T.W.W. supervised the whole study.





